CVS Health 12 Hour Sinus Relief Nasal Mist .5 oz bottle has been recalled after the product was found to have had microbiological contamination identified as Pseudomonas aeruginosa, according to the Food and Drug Administration.
The product is used as a nasal decongestant and is packaged in a 0.5 fluid ounce bottle that is placed in an individual folding carton.
16,896 units were released nationwide with UPC code 50428432365.
The affected CVS Health 12-Hour Sinus Relief Nasal Mist lot is Lot # 173089J, EXP 09/19. The lot number can be found on the side panel of the carton.
According to the recall release, "Repetitive use of a nasal spray containing a gram-negative pathogen can potentially lead to colonization and subsequent infection which can be life-threatening in certain patient populations, such as those with cystic fibrosis or immuno-compromised. To the best of Product Quest's knowledge, the company has not received any reports of adverse events related to this recall."
Product Quest is notifying its customers via oral and written communication and is arranging for the return or replacement of all recalled products.
Anyone that has the affected spray is being asked to stop using the product and return it to the place of purchase or throw it away.
Consumers with questions regarding this recall can contact Product Quest Manufacturing LLC at (386) 239-8787, Monday through Friday from 8 am to 4 pm, EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
=========================
Pseudomonas aeruginosa in Healthcare Settings
What is a Pseudomonas infection?
Pseudomonas infection is caused by strains of bacteria found widely in the environment; the most common type causing infections in humans is called Pseudomonas aeruginosa.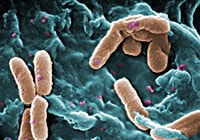 Pseudomonas aeruginosa
Pseudomonas aeruginosaWhat types of infections does Pseudomonas aeruginosa cause?
Serious Pseudomonas infections usually occur in people in the hospital and/or with weakened immune systems. Infections of the blood, pneumonia, and infections following surgery can lead to severe illness and death in these people.However, healthy people can also develop mild illnesses with Pseudomonas aeruginosa, especially after exposure to water. Ear infections, especially in children, and more generalized skin rashes may occur after exposure to inadequately chlorinated hot tubs or swimming pools. Eye infections have occasionally been reported in persons using extended-wear contact lenses.
Who is at risk for infection?
Patients in hospitals, especially those on breathing machines, those with devices such as catheters, and patients with wounds from surgery or from burns are potentially at risk for serious, life-threatening infections.How is Pseudomonas aeruginosa spread?
In hospitals, where the most serious infections occur, Pseudomonas can be spread on the hands of healthcare workers or by equipment that gets contaminated and is not properly cleaned.How can Pseudomonas infections be prevented?
In the hospital, careful attention to routine infection control practices, especially hand hygiene and environmental cleaning, can substantially lower the risk of infection.Outside the hospital, avoid hot tubs or pools that may be poorly maintained, and keep contact lenses, equipment, and solutions from becoming contaminated.
How are Pseudomonas infections treated?
Pseudomonas infections are generally treated with antibiotics. Unfortunately, in hospitalized patients, Pseudomonas infections, like those caused by many other hospital bacteria, are becoming more difficult to treat because of increasing antibiotic resistance. Selecting the right antibiotic usually requires that a specimen from a patient be sent to a laboratory to test to see which antibiotics might still be effective for treating the infection.Multidrug-resistant Pseudomonas can be deadly for patients in critical care. An estimated 51,000 healthcare-associated P. aeruginosa infections occur in the United States each year. More than 6,000 (13%) of these are multidrug-resistant, with roughly 400 deaths per year attributed to these infections. Multidrug-resistant Pseudomonas was given a threat level of serious threat in the CDC AR Threat report.
What is the CDC doing to monitor, prevent, and/or help treat infections?
The CDC conducts surveillance, prevention, and outreach activities to help prevent infections and to ensure that serious infections stay susceptible to commonly-used antibiotics and do not become resistant and hard to treat. CDC employees work closely with state and local health departments, Federal agencies, healthcare providers, and consumers of healthcare to help educate people about Pseudomonas infections, and antibiotic resistance, and to encourage prevention activities and healthy behaviors to prevent antibiotic resistance.In early 2014, CDC made a request to states to send relevant isolates so that CDC microbiologists could further characterize carbapenem resistance mechanisms in Pseudomonas. As part of this, CDC was able to confirm several plasmid-mediated carbapenemases among Pseudomonas.



