Perfluorooctane Sulfonate (PFOS) in Drinking Water
Prepared by the Federal-Provincial-Territorial Committee on Drinking Water
Consultation period ends September 2nd, 2016
Table of Contents
- Purpose of consultation
- Part I. Overview and Application
- 1.0 Proposed guideline
- 2.0 Executive summary
- 3.0 Application of the guideline
- Part II. Science and Technical Considerations
- 4.0 Identity, use and sources in the environment
- 5.0 Exposure
- 6.0 Analytical methods
- 7.0 Treatment technology
- 7.1 Municipal scale
- 7.2 Residential scale
- 8.0 Kinetics and metabolism
- 9.0 Health effects
- 9.1 Effects in humans
- 9.2 Effects on experimental animals
- 9.3 Mode of action
- 10.0 Classification and assessment
- 11.0 Rationale
- 12.0 References
- Appendix A: Reported full-scale drinking water treatment plant PFOS/PFOA removal data
- Appendix B: List of acronyms
- Appendix C: Provincial and territorial anticipated impacts
Purpose of consultation
The Federal-Provincial-Territorial Committee on Drinking Water (CDW) has assessed the available information on PFOS with the intent of developing a drinking water guideline and guideline technical document on PFOS in drinking water. The purpose of this consultation is to solicit comments on the proposed guideline, on the approach used for its development and on the potential economic costs of implementing it, as well as to determine the availability of additional exposure data.This document proposes a maximum acceptable concentration (MAC) of 0.0006 mg/L (0.6 µg/L) for PFOS in drinking water, based on liver effects in rats. The document is based on currently available scientific studies and approaches. It incorporates a detailed mode of action analysis to establish a proposed guideline for PFOS. It provides exposure information as well as analytical methods and treatment technologies that may be effective for PFOS removal at the municipal and residential scales.
The CDW has requested that this document be made available to the public and open for comment. Comments are appreciated, with accompanying rationale, where required. Comments can be sent to the CDW Secretariat via email at water_eau@hc-sc.gc.ca. If this is not feasible, comments may be sent by mail to the CDW Secretariat, Water and Air Quality Bureau, Health Canada, 3rd Floor, 269 Laurier Avenue West, A.L. 4903D, Ottawa, Ontario K1A 0K9. All comments must be received before September 2nd, 2016.
Comments received as part of this consultation will be shared with the appropriate CDW member, along with the name and affiliation of their author. Authors who do not want their name and affiliation shared with their CDW member should provide a statement to this effect along with their comments.
It should be noted that this guideline technical document on PFOS in drinking water will be revised following evaluation of comments received, and a drinking water guideline will be established, if required. This document should be considered as a draft for comment only.
Part I. Overview and Application
1.0 Proposed guideline
A maximum acceptable concentration (MAC) of 0.0006 mg/L (0.6 µg/L) is proposed for PFOS in drinking water.2.0 Executive summary
PFOS is a man-made compound that does not occur naturally in the environment. It is no longer manufactured, imported, sold, offered for sale or used in Canada, but is still found in the environment because of its extremely persistent nature. PFOS was used for water, oil and/or stain resistance on surface and paper-based applications, such as rugs and carpets, fabric and upholstery. It was also used in specialized chemical applications, such as fire-fighting foams, hydraulic fluids, and carpet spot removers.This guideline technical document reviews and assesses all identified health risks associated with PFOS in drinking water. It incorporates available studies and approaches and takes into consideration the availability of appropriate analytical methods and treatment technology. Based on this review, the proposed drinking water guideline for PFOS is a maximum acceptable concentration (MAC) of 0.0006 mg/L (0.6 µg/L).
During its fall 2015 meeting, the Federal-Provincial-Territorial Committee on Drinking Water reviewed the guideline technical document on PFOS and gave approval for this document to undergo public consultation.
2.1 Health effects
The carcinogenicity of PFOS has not been evaluated by the International Agency for Research on Cancer (IARC). Some cancer effects were observed in humans after exposure to PFOS, but no clear links could be made due to various study limitations. Tumours were observed in the liver, thyroid, and mammary gland of rats following long term exposure to PFOS. Non-cancer effects occurring at the lowest level of exposure to PFOS in animals include effects on the immune system, liver effects, effects on the thyroid and changes in serum lipid levels.Both cancer and non-cancer endpoints were considered in the derivation of the proposed MAC for PFOS in drinking water. The non-cancer approach, based on liver effects in rats, was used to calculate a proposed MAC that is protective of human health from both cancer and non‑cancer effects.
2.2 Exposure
Canadians can be exposed to PFOS through its presence in food, consumer products, dust, and drinking water. Exposure is mainly from food and consumer products, however, the proportion of exposure from drinking water can increase in individuals living in areas with contaminated drinking water. Although PFOS is not regularly monitored at water treatment plants in Canada, the analysis has been performed for a few locations. When detected in drinking water, it is usually found below 1 ng/L.2.3 Analysis and treatment
To date, the United States Environmental Protection Agency has not approved any analytical methods for the analysis of PFOS in drinking water. There are some methods that can be used to measure PFOS in drinking water at levels well below the proposed MAC. However, they require good quality control procedures to get accurate results.The selection and effectiveness of a treatment strategy for PFOS removal is driven by several factors, including source water chemistry, concentration of PFOS and/or other perfluoroalkyl substances and pre-existing treatment processes. Conventional treatment is not effective for PFOS removal. Other treatment methods are promising, although full-scale studies are limited. Granular activated carbon (GAC) adsorption can achieve treated water concentrations of PFOS below the proposed MAC. However, proper operation of the system is essential to ensure that the performance of GAC is not affected by the presence of natural organic matter in the source water. Membrane filtration techniques (reverse osmosis and nanofiltration) and anion exchange may also be effective. Although there are no residential treatment devices certified to remove PFOS, it is expected that the same treatment technologies would also be effective at the residential scale.
3.0 Application of the guideline
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority in the affected jurisdiction.3.1 Monitoring
PFOS is typically found in groundwater and surface water contaminated by discharges from industrial facilities; discharge from wastewater treatment plants treating domestic and industrial waste; storm water runoff; land application of biosolid amended soils as well as groundwater and surface water sources that are or may have been impacted by aqueous film‑forming foam (AFFF) (i.e., fire-fighting foams). Like other groundwater contaminants, PFOS can reach drinking water wells through migration of a contaminated groundwater plume. It can also reach groundwater from air emissions of industrial facilities. Particle-bounded volatile perfluoroalkyl substances (PFASs) may be carried by wind from disposal sites and deposited on the land or the surface water thus explaining the presence of these anthropogenic chemicals in remote locations and, in waters not impacted by a point source. PFOS migrates very slowly through the soil to groundwater and may not be detected in the source water for years.Utilities should characterize their source water to assess PFOS concentrations. In source waters where PFOS is present at levels exceeding the proposed MAC, quarterly monitoring of surface water and semi-annual monitoring of groundwater should be conducted.
The monitoring frequency of the treated water depends on the treatment technology the utility employs. Utilities that use a granular activated carbon (GAC) system for PFOS removal may want to enhance monitoring of the treated water in order to assess the performance of the GAC system and to determine the timing of the regeneration. Utilities may consider reduced monitoring when they have data indicating that PFOS does not occur in the source water. However, if the main source of groundwater contamination is suspected to be from the use of AFFF, utilities may want to consider monitoring for other perfluorinated alkyl acids PFAAs (i.e., shorter chain compounds such as perfluorobutanoic acid and perfluorobutane sulfonate). These other PFAA compounds are likely to co-occur at AFFF-impacted sites and are typically more mobile. As such, they can serve as an early warning sign of PFOA and PFOS contamination of a groundwater source. Utilities should be aware that ozone or AOPs may oxidize polyfluorinated precursor chemicals present in the raw water, which could result in an increased concentration of PFOS in the finished water.
Part II. Science and Technical Considerations
4.0 Identity, use and sources in the environment
Perfluorooctane sulfonate (PFOS) is an anthropogenic compound with a chain length of 8 perfluorinated carbons. PFOS, its salts and its precursors form part of a larger chemical class of fluorochemicals typically referred as perfluorinated alkyl acids (PFAAs). PFOS can occur under several forms, including the acid (C8HF17SO3; 500.03 g/mol; CAS number 1763-23-1), the potassium salt (K+PFOS; 538.23 g/mol; CAS number 2795-39-3), the ammonium salt (NH4+PFOS; CAS number 29081-56-9), the diethanolamine salt (C8HF17SO3NH; CAS number 70225-14-8), and the lithium salt (Li+PFOS; 29457-72-5). The main synonyms of PFOS are 1-perfluorooctanesulfonic acid, heptadecafluoro-1-octanesulfonic acid, heptadecafluorooctan-1-sulphonic acid, perfluorooctane sulfonate, perfluorooctylsulfonic acid and 1-octanesulfonic acid (ATSDR, 2009).PFOS is soluble in water, with solubility values reported at 570 mg/L and 519 mg/L (at 20°C) in pure water (OECD, 2002; Brooke et al., 2004) and at 370 mg/L in freshwater (OECD, 2002); the solubility decreases when the water salt content increases (OECD, 2002). The solubility depends on the acid dissociation constant (pKa) of the acid form; the pKa value for PFOS has been estimated at -3.27 (no direct measurement of the pKa has been located) and PFOS is considered to be a strong acid, suggesting that the environmental partitioning of PFOS will be dominated by the anionic form (Brooke et al., 2004).
PFOS contains both hydrophobic and hydrophilic functional groups and is thus expected to behave differently than traditional hydrophobic chemicals. Due to its surfactant properties, the octanol:water partition coefficient (LogKow) cannot be determined directly because multiple layers are formed in octanol/water. Moreover, the parameters usually estimated from the Kow (e.g. Koc, Kd, bioconcentration factor) cannot be calculated using this method (OECD, 2002), nor using conventional quantitative structure-activity relationship models (QSAR) (Beach et al., 2006).
PFOS is essentially non-volatile, with a vapour pressure of 3.27 × 10-9 atm at 20°C (OECD, 2002; Brooke et al., 2004; ATSDR, 2009); its Henry's Law constant was estimated at approximately 3.1 × 10-9 atm-m3/mol. Attempts to measure the air-water partition coefficient using the potassium salt indicate no volatilization to any measurable extent; the air-water partition coefficient was thus considered to be < 2 × 10-6, and to be essentially zero (OECD, 2002; Brooke et al., 2004). Nevertheless, some of the PFOS-containing substances have considerably higher vapour pressure and are more likely to be volatile to some extent. This may allow the wider transport of potential PFOS precursors through the air than is possible for PFOS itself (Brooke et al., 2004).
The principal applications for PFOS were for water, oil and/or stain resistance for use on surface and paper-based applications, such as rugs and carpets, fabric and upholstery. PFOS was also used in specialized chemical applications, such as fire-fighting foams, hydraulic fluids, carpet spot removers, mining and oil well surfactants and other specialized chemical formulations (OECD, 2002; Health Canada, 2006). PFOS was produced in the U.S. until 2002, when the 3M Company phased out its PFOS production (ATSDR, 2009). Although there are no known Canadian manufacturers of PFAAs, including PFOS, almost 600,000 kg of PFAAs were imported into Canada between 1997 and 2000 (PFOS represented a very small proportion of this total) (Health Canada, 2006). In 2009, PFOS and its salts were added to the Canadian Virtual Elimination List compiled under subsection 65(2) of CEPA 1999, as required by subsection 3(1) of the Act (SOR/2009-15) (Government of Canada, 2009). The Canadian regulations prohibit the manufacture, import, sale, offer for sale and use of PFOS or products containing PFOS, unless incidentally present, with certain exemptions for aviation hydraulic fluids under certain conditions, and some products used in photographic or photolithographic process (Government of Canada, 2008).
4.1 Sources to water
A source of PFAAs in water is the discharge of aqueous film-forming foam (AFFF) for extinguishing fires. Discharge of AFFF was presumed to have resulted in increased levels of PFOS in water surrounding the Toronto International Airport, based on spatial and temporal trends of PFAAs in water (Awad et al., 2011). However, as of 2013, most uses of AFFF containing PFOS at concentrations of >0.5 ppm have been banned (Government of Canada, 2008). Data supporting the possibility of contamination in the vicinity of firefighting training areas include measurements of elevated PFOS concentrations in groundwater near a Michigan air force base (Moody et al., 2003), at a firefighting training ground in Australia (Baduel et al., 2015), and in private drinking water wells proximate to an industrial site in Cologne, Germany (Weiß et al., 2012).Elevated PFOS concentrations measured in surface water downstream from fluorochemical manufacturing plants have also been used as indications of the potential for industrial sources of PFOS in water (Hansen et al., 2002).
Mass balance studies of PFAAs at wastewater treatment plants commonly report similar or higher PFOS concentrations in the effluent in comparison to the raw influent, suggesting that the degradation of other fluorinated organic compounds (i.e. fluoropolymers) into PFOS may take place during wastewater treatment (Clarke and Smith, 2011). This also indicates that conventional wastewater treatment plants are not effective for removal of PFAAs (Ahrens, 2011).
Although measures are in place in North America and Europe to restrict the production, use and/or the major exposure risks to PFOS, the ubiquitous use of PFAAs within the built environment still causes their transfer to biosolids (sludge) (Clarke and Smith, 2011). The use of biosolids as fertilizers may thus represent a source of soil and water contamination with PFOS (Clarke and Smith, 2011). Drinking water contamination was reported after the widespread use of soil conditioner mixed with industrial waste containing PFAAs (e.g., 8,600 ng PFOS/g d.w.) in the region of Arnsberg, North Rhine Westphalia, Germany (Hölzer et al., 2008). PFOS was also measured in surface and well water in Decatur, Alabama, after biosolids from a municipal wastewater treatment plant (at which waste from local fluorochemical facilities were received) were applied in agricultural fields (Lindstrom et al., 2011).
4.2 Environmental fate
The elevated water solubility of PFOS and the negligible volatility of its ionized species suggest that PFOS species will partition primarily to the aquatic environment.PFOS is a strong acid and most likely forms strong bonds in soils, sediments, and sludge via chemisorption mechanism (3M Company, 2001; Brooke et al., 2004; Beach et al., 2006), with greater adsorption under anaerobic conditions than aerobic conditions (Beach et al., 2006). PFOS does not partition into lipids but instead binds to certain proteins in animals (Beach et al., 2006). PFOS bioaccumulates in tissues of aquatic and terrestrial living organisms including humans. Data for the marine food web from the Eastern Canadian Arctic (from 1996 to 2002) indicate that PFOS biomagnifies through the entire food web with a trophic magnification factor of 3.1 (Butt et al., 2010).Under environmental conditions, PFOS does not hydrolyze, photolyze or biodegrade, and it is considered extremely persistent in the environment (OECD, 2002; Beach et al., 2006; Environment Canada and Health Canada, 2012). The estimated half-lives for PFOS (as the potassium salt) are > 41 years in water (ATSDR, 2009) and 114 days in the atmosphere (Brooke et al., 2004); the indirect half-life of PFOS was estimated (using an iron oxide photoinitiator model) to be ≥ 3.7 years (OECD, 2002; Beach et al., 2006). No study was able to demonstrate the biodegradation of PFOS under aerobic or anaerobic conditions (Beach et al., 2006) and PFOS is considered resistant to microbial degradation (Health Canada, 2006). Moreover, the abiotic degradation of certain PFOS precursor molecules can lead to PFOS as the end stage metabolite product (Martin et al., 2010). Hydrolysis rates (varying from days to weeks) for PFOS precursors are provided in 3M Company studies (Mendel, 1977; 3M Company, 1996; Hatfield, 1999).
5.0 Exposure
Canadians can be exposed to perfluorinated compounds present in food, consumer products, dust, and drinking water. The major sources of PFAAs are expected to be food and consumer products, including solution-treated carpeting and treated apparel (Tittlemier et al., 2007); however, the proportion of exposure from drinking water can increase in individuals living in areas with contaminated drinking water.The estimated total daily intake of PFAAs (estimates not provided for individual PFAAs) in Canadians was reported to be 410 ng/day for the general population of Canada (Tittlemier et al., 2007). Drinking water ingestion contributed only a minor amount to the overall estimated exposure (estimated at 0.3 ng/day). Although some exposure data are available, they are considered insufficient to justify modifying the default allocation factor for drinking water of 20%. Twenty percent is the default allocation factor for drinking water which is used as a "floor value" when drinking water is not a major source of exposure (Krishnan and Carrier, 2013); therefore, this value is applicable for PFOS, even though water is expected to be only a minor contributor to PFOS exposure for the general population.
5.1 Water
Although PFOS is not regularly monitored at drinking water treatment plants in Canada, the analysis has been performed for a few locations. PFOS was not detected (method detection limit [MDL]= 0.85 ng/L) in raw or finished water from samples obtained in 2012 from two drinking water treatment plants in Calgary (Alberta Environment and Water, 2013). In Quebec, raw and treated water samples were obtained monthly between April 2007 and March 2008 from seven sites (a total of 84 samples each of raw and treated water). PFOS was detected in 52% of treated samples (MDL of 0.3-0.6 ng/L), with a median value of 1.0 ng/L. The detection rate and median concentrations were higher in treated water than in raw water, for which the detection rate and median were 40% and <1 ng/L, respectively (Ministère du Développement durable, de l'Environnement, de la Faune et des Parcs, 2012). The reported PFOS concentration in 5 tap water samples from Niagara-on-the-Lake, Ontario was 3.3 ng/L (Mak et al., 2009).As part of a national survey of emerging contaminants in drinking water (including PFOS) conducted by Health Canada, treated and raw water were monitored in winter and summer at 35 locations in 2009 and 30 locations in 2010. In the four sampling periods, only one sample contained PFOS above the method detection limit (MDL) of 0.077 ng/L; the PFOS concentration in that sample was 0.082 ng/L, and was obtained in the winter of 2009 (Health Canada, 2013a).
5.2 Food
Food is generally considered to be the main source of PFOS exposure for the majority of the Canadian population, but exposure from food is still well below what is considered unsafe to humans. PFOS was measured in a selection of Canadian food composite samples (samples from the Canadian Total Diet Study (TDS) conducted in 2004 and additional samples collected between 1992 and 2001) to estimate dietary intake (Tittlemier et al., 2007). PFOS was detected in 7 out of 54 food composites (average detection limit: 0.5 ng/g). The quantified concentrations ranged from 2.0 to 2.7 ng/g wet weight (w.w.; in marine and freshwater fish, ground beef and beef steak). Concentrations lower than the limit of quantitation (LOQ) were reported for microwave popcorn, luncheon meats and cold cuts, and freshwater fish. Values were used to estimate the average dietary daily exposure of Canadians; food was estimated to contribute 250 ng/day of perfluorinated compounds, of which approximately 110 ng was attributed to PFOS (Tittlemier et al., 2007).Store-bought and restaurant foods commonly consumed by Canadians were collected in Whitehorse (Yukon Territory, Canada) in 1998 and analyzed for PFAAs (Ostertag et al., 2009a). PFOS was detected in only 2 samples (regular and processed cheese) and quantifiable in one sample (processed cheese: 1.14 ng/g w.w.; Ostertag et al., 2009a).
The marine ecosystem of the Eastern Canadian Arctic represents a source of food for the local population. In this region, the PFOS levels (on a w.w. basis) were reported to range from 0.28 to 1.8 ng/g w.w. in zooplankton and invertebrates, from 1.3 to 1.4 ng/g w.w. in fish, and from 2.4 to 122 ng/g in marine mammals (whales and pinnipeds) (Butt et al., 2010). The concentrations of PFAAs in the traditional foods of Inuit in Northern Canada were measured in order to estimate their dietary exposure (Ostertag et al., 2009b). PFOS was detected in 39% of the 68 traditional food samples collected from Chesterfield Inlet, Igloolik, Pond Inlet and Qiqiktarjuak in Nunavut, between 1997 and 1999. PFOS was detected in both aquatic food (0.1-7.6 ng/g in ringed seal, polar bear (meat), beluga, narwhal, bearded seal, walrus, eider and black duck, or lake trout) and terrestrial food (5.0 ng/g in baked caribou liver, 0.1-0.2 ng/g in caribou bone marrow, heart, blood, kidney, stomach, tongue or meat). PFOS concentrations in the other samples (arctic char, seaweed, clams, ptarmigan, artic hare, snow goose, berries) were below the detection limit (˂0.1 to ˂0.5 ng/g) (Ostertag et al., 2009b).
An Australian study quantified PFAAs in food packaging and polytetrafluoroethylene (PEFT) sealant tape. PFOS was not detected in any of these samples, including in microwave popcorn bags (Dolman and Pelzing, 2011).
5.3 Air
In an assessment designed to estimate total daily intake of perfluorinated compounds in Canadians, the inhalation intake of PFOS was considered negligible due to its low volatility (Tittlemier et al., 2007).The levels of PFAAs in outdoor air were determined in a Canadian study conducted in 2007 in Vancouver (Shoeib et al., 2011). PFOS samples were collected using outdoor passive samplers deployed in residential yards for approximately 3 months. PFOS levels were below the detection limit (< 0.02 pg/m3) in all samples (n = 6) (Shoeib et al., 2011). In another study, PFOS was detected in 4 out of 8 air samples (particulate-phase) collected over Lake Ontario, at levels varying between 2.5 and 8.1 pg/m3 (PFOS remained undetected in gaseous-phase samples) (Boulanger et al., 2005). PFOS was also detected in the Canadian Arctic (Resolute Bay, Nunavut) with a mean concentration of 5.9 pg/m3 in the gas and particulate phase of atmospheric air (2004 sampling) (Fromme et al., 2009; Butt et al., 2010).
In indoor air, the levels of PFOS mainly depend on PFOS concentration in air particulates and are thus related to PFOS levels in indoor dust, as well as the number, type and age of the potential sources (e.g. carpeting, furniture and paint) (Fraser et al., 2012). To date, data on indoor air concentrations of PFOS are limited to those reported in the aforementioned residential study (Shoeib et al., 2011). The authors collected PFOS in indoor air using passive samplers deployed for approximately 4 weeks in bedrooms of 59 participants. PFOS levels (available for 39 homes) were below the detection limit (< 0.02 pg/m3) in all samples (Shoeib et al., 2011).
5.4 Consumer products
Owing to the use patterns of PFOS, human exposure to PFOS would likely result from contact with, or the use of, certain consumer products (Health Canada, 2006). Estimates of the contribution of solution-treated carpeting and treated apparel to Canadians' daily intakes of perfluorinated compounds were 120 ng/day and 12 ng/day, respectively (Tittlemier et al., 2007). PFOS has been measured in a variety of consumer products, including paint, printed circuit boards, carpet, leather, non-stick ware, and aqueous firefighting foams (Herzke et al., 2012).5.5 Soil and household dust
Estimated contribution of dust to Canadians' daily intakes of perfluorinated compounds was 28 ng/day (Tittlemier et al., 2007). The study did not estimate the total daily contribution of soil to perfluorinated compound exposure.PFOS concentrations in dust in 67 Ottawa homes were between <4.6 and 5,065 ng/g, with a median value of 38 ng/g and a mean value of 444 ng/g (Kubwabo et al., 2005). House age and fraction of floor covering were reported to be significantly correlated with the concentration of PFAAs in dust-older houses and those with smaller fractions of the floor covered with carpet were characterized by lower concentrations of PFAAs (Kubwabo et al., 2005). In another Canadian study conducted in the City of Vancouver, PFOS was detected in all household dust samples analyzed for this compound (n = 132). The PFOS concentrations ranged from 1.5 to 4,661 ng/g (median: 71 ng/g, mean: 280 ng/g) (Shoeib et al., 2011).
No study reporting background PFOS levels in soils was found. Some data are available in soils surrounding perfluorochemical industrial facilities (as reviewed by ATSDR, 2009).
5.6 Human biomonitoring data
The Canadian Health Measures Survey (CHMS), Cycle 1 (2007-2009) indicates that PFOS plasma levels in adult males (geometric mean [GM]: 11.13 ng/mL; 95% CI: 10.03-12.36, 95th percentile: 31.31 ng/mL, n=1,376) are higher than in adult females (GM: 7.07 ng/mL; 95% CI: 6.30-7.93, 95th percentile: 20.05 ng/mL, n=1,504) (Health Canada, 2010). This effect persisted in Cycle 2 of the study (2009-2011), which observed a decline in plasma concentrations compared with Cycle 1 (Males-GM: 8.3 ng/mL, 95% CI: 7.4-9.3, 95th percentile: 19 ng/mL, n=511; Females-GM: 5.7 ng/mL, 95% CI: 4.9-6.6, 95th percentile: 19 ng/mL, n=506) (Health Canada, 2013b).PFOS was detectable in all serum samples (n = 86) collected from 2006-2008 in a study of Inuit children attending childcare centers in Nunavik (Turgeon O'Brien et al., 2012). The geometric mean of PFOS in serum was 3.369 ng/mL with a range of 0.93-31 ng/mL. A separate study of 621 Nunavik Inuit adults reported PFOS serum levels of 0.480 to 470 ng/mL (GM: 18.28 ng/mL, 95% CI: 17.19-19.44 ng/mL) (Dallaire et al., 2009). Other Canadian studies have reported similar levels of PFOS in serum ranging from 3.7 to 63.1 ng/mL (Tittlemier et al., 2004; Kubwabo et al., 2004). Similar results have been shown elsewhere-the overall range of mean PFOS concentrations (in males or females) was from 1.7 to 73.2 ng/mL in serum samples collected in 10 countries (Kannan et al., 2004). PFOS concentrations in human serum/plasma collected worldwide (America, Asia, Australia, and Europe) over a period from 1998-2007 reported mean concentrations ranging from 2.1 to 62 ng/mL (Ingelido et al., 2010). In the U.S., data from the National Health and Nutrition Examination Survey (NHANES) for the period 2007-2008 indicate a median serum PFOS level of 13.6 ng/mL in the general population (≥ 12 years of age), with a downward temporal trend noted for the period 1999-2008 by Kato and colleagues (2011).
5.7 Multi-route exposure through drinking water
The multi-route exposure assessment process applied is not applicable for PFOS, due to the compound's high molecular weight and low volatility (Krishnan and Carrier, 2008); therefore, the relative contributions of exposure to PFOS from both inhalation and dermal routes during showering and bathing were not estimated. Based on the high molecular weight of 500.03 g/mol and the ionic properties of PFOS at pH levels typical in drinking water, volatility and dermal penetration are expected to be low. Moreover, dermal permeability coefficients estimated in in vitro studies predict that PFOS is impermeable to skin under typical conditions (Fasano et al., 2005; Franko et al., 2012). Consequently, exposure to PFOS via inhalation and dermal routes during showering or bathing is expected to be negligible.6.0 Analytical methods
To date, the United States Environmental Protection Agency (U.S. EPA) has not approved any analytical methods for the analysis of PFOS in drinking water. There are some methods that can be used to measure PFOS in drinking water at levels well below the proposed MAC. However, they require good quality control procedures to produce accurate results.6.1 Available methods
U.S. EPA Method 537 ver. 1.1, International Standard Organization (ISO) Method, 25101 (ISO, 2009) and 3M Method ETS-8-154.3 (3M Company, 2008) can all be used for the analysis of PFOS in drinking water (3M Company, 2008; ISO, 2009; U.S. EPA, 2009). All methods use a solid phase extraction (SPE) technique followed by a liquid chromatograph (LC) coupled to electrospray ionization (ESI) tandem mass spectrometry (MS/MS) operated in negative ion mode. For the purpose of trace quantitation of PFOS in drinking water, the chromatographic conditions are selected such that all isomers (linear and branched) are co-eluted together.In the EPA method, a water sample is fortified with labelled internal standards and passed through a SPE cartridge to extract target analytes in addition to their corresponding internal standards. The compounds are eluted from the SPE cartridge, concentrated and injected into a LC-MS/MS. The mass spectra and retention times of the analytes are identified by comparison to internal standards. The method detection limit (MDL) of PFOS is 1.4 ng/L (0.0014 µg/L) and the Lowest Concentration Minimum Reporting Level (LCMRL) is 6.5 ng/L (0.0065 µg/L) (U.S. EPA, 2009a). PFOS has been included in the third Unregulated Contaminant Monitoring Rule (UCMR3), which stipulates that using Method 537 ver. 1.1, an MRL of 40 ng/L (0.04 µg/L) for PFOS must be achieved and reported by the utilities during monitoring (U.S. EPA, 2012b).
The results of an inter-laboratory trial (Taniyasu et al., 2013), conducted in 2006, were used to establish whether ISO Method 25101 was reliable for the analysis of PFOS and PFOA in environmental water samples, including drinking water. The intra- and inter laboratory precisions were in the range of 3-4% and 16-27%, respectively for PFOS for all environmental water samples analyzed. The recovery of the internal standards for PFOS ranged from 90 to 96%. These results confirmed that this analytical method was reliable and can be used for the analysis of PFOS in environmental water samples. The method uses SPE, LC-MS/MS and is applicable for the quantification of the linear and branched isomers of PFOS and PFOA. The branched isomers can be separated from the linear isomers by using specific chromatographic column and optimized conditions. ISO Method 25101 was found to be appropriate for determination of PFOS levels in unfiltered samples of drinking water, groundwater and surface water with concentrations in the range of 2 - 10,000 ng/L (0.002 - 10 µg/L) (ISO, 2009).
Method (ETS-8-154.3) was developed and validated by 3M for PFOS analysis in drinking water, groundwater and surface water samples. The analytical steps are similar to EPA Method 537 Ver 1.1 and the method has an LOQ of 25 ng/L (0.025 µg/L) for PFOS (3M Company, 2008).
6.2 Analytical challenges
In spite of the significant improvements in the analytical methods for the determination of PFASs in environmental water samples, challenges, uncertainties and drawbacks still remain. Major challenges associated with the trace quantitation of PFASs included matrix effects and a background contamination in the analytical blanks. In order to generate accurate data, quality control (QC) procedures (matrix spikes, duplicates, spike-recovery experiments, surrogate recovery checks) are critical. In addition, the use of isotope-labelled internal standards is a standard practice and must be used in the analysis of PFASs.6.2.1 Matrix effect
Although LC-MS/MS is a highly selective and sensitive technique, it is susceptible to matrix effects which is one of the major uncertainties in the trace quantitation of PFOS in environmental water samples (Martin et al., 2004; Yamashita et al., 2004; Taniyasu et al., 2005; van Leeuwen et al., 2006; Arsenault et al., 2008). Matrix effects result from the co-extracted components from the sample, which affect the signal intensity of the target analyte and either suppress or enhance the spectral signal. The extent of the matrix interference varies, depending on the nature of the samples. Although the matrix interferences are negligible for drinking water and groundwater (ISO, 2009), the PFOS quantification requires efficient extraction and clean-up procedures. The aim of these procedures is to separate the compounds in the sample by their chemical and physical properties, to concentrate the target analyte and to purify the extract prior to the instrumental determination. The most frequently used technique for the extraction of PFASs from drinking water samples includes SPE cartridges with different packing material such as reverse phase (C18) cartridge (Loewen et al., 2005; Wolf and Reagen, 2011; Zainuddin et al., 2012), mixed hydrophobic/polar (Oasis HLB) cartridges (Yamashita et al., 2004; Taniyasu et al., 2005; Villaverde-de-Saa et al., 2015) and a weak anion exchange (WAX) cartridges (Taniyasy et al., 2005; 2013). Several studies conducted a liquid-liquid extraction (LLE) technique to extract and concentrate PFASs in different environmental aqueous matrices prior to LC-MS/MS (Gonzales-Barreiro et al., 2006; Szostek et al., 2006; Backe et al., 2013). A laboratory study (Gonzales-Barreiro et al., 2006) used an LLE to extract 7 PFASs (C6-C12) from tap water. The recovery of the PFASs with a carbon chain greater than C7 was in the range 80-93%. The authors indicated that the method was less efficient in extracting short-chained PFASs when compared to the SPE technique (Gonzales-Barreiro et al., 2006).The clean-up procedures involved a washing step after the sample enrichment on the SPE cartridge and a filtration to remove solids from the final extract (Yamashita et al., 2004; Larsen and Kaiser, 2007; van Leeuwen and Boer, 2007). Care should be taken to avoid contamination of the extract or losses of PFASs during the clean-up procedures. Prior to a SPE, a sample pre‑treatment (filtration) may be required to facilitate extraction or to remove matrix constituent that will interfere with analyses (van Leeuwen and Boer, 2007; Ding et al., 2012).
The most suitable approach to assist in the quantification of PFASs is to use of isotopically-labelled internal standards (isotope dilution). It is important that the appropriate isotope-labelled internal standards are used for the quantitation of the corresponding native compound. Isotope-labelled internal standards will have the same retention time as the target analytes (excluding isomeric separation) and the monitoring of their signals will determine whether the analytes signal are suppressed or enhanced. The application of surrogates or isotopically-labelled internal standards early in the sampling or the sample preparation steps will compensate for the inefficiency/losses in the extraction and other sample preparation steps (Martin et al., 2004; Villagrassa et al., 2006; Larsen and Kaiser, 2007). Wolf and Reagen (2011) reported that an addition of isotope-labelled internal standards prior to sample collection simplified the sample preparation procedures. The method demonstrated an accuracy of 109% and a precision of 10% for PFOS in laboratory Milli-Q water samples (Wolf and Reagen, 2012). If isotope-labelled internal standards are not available, a standard addition quantitation, which involves spiking known quantities of a standard into the sample, is an alternative to use when matrix effects are unavoidable (Weremiuk et al., 2006; Furdui et al., 2007; van Leeuwen et al., 2009).
The use of MS/MS for analysis of PFOS enables the detection of product (daughter) ions. Although the transition m/z 80 (product ion SO3-) is the most abundant product ion used for determination of PFOS, m/z 99 (product ion FSO3-) is also used for PFOS identification (ISO, 2009; U.S. EPA, 2009a).
6.2.2 Background contamination in the analytical blanks
A known source of background contamination is the presence of fluoropolymers, such as polytetrafluoroethylene (PTFE) and perfluoroalkoxy compounds in various laboratory consumables. Ammonium perfluorooctanoate and ammonium perfluorononanoate are used as fluoropolymer processing aids and are common components in the laboratory products. These fluoropolymers may lead to quantifiable background levels in the analytical blanks especially when quantifying trace levels in water samples. Contacts with such laboratory materials and products during analysis of PFOS should be avoided (Martin et al., 2004; Yamashita et al., 2004; ISO, 2009).Yamashita et al. (2004) studied the sources of background contamination at various analytical steps, including sample collection, extraction and sample clean up prior to the instrumental analysis. Polypropylene bottles used for sample collection and storage, in addition to different types of SPE cartridges and purified reagent water, were found to be sources of PFASs contamination in the analytical blanks. Taniyasu et al. (2005) and Berger et al. (2011) found that the polypropylene containers are unsuitable for collection and storage of water samples intended for analysis of long-chain perfluorocarboxylic acids (PFCAs) such as perfluoroundecanoic and perfluorododecanoic acids, because of the adsorption of the compounds on the containers' surface. The authors recommended the use of high density polyethylene or glass containers. However, ISO method 25101 and EPA Method 537 recommended against the use of glassware for sampling due to the potential adsorption of PFOS on the walls (ISO, 2009; U.S. EPA, 2009a). The storage and sample preservation steps prior to the instrumental analysis should prevent changes in composition of the sample matrix and the concentration of the analyte (van Leeuwen et al., 2007).
SPE cartridges can also be a source of contamination and the U.S. EPA (2009a) recommends that SPE devices be tested prior to using them for analysis to ensure that there is no contamination of the sample. Several studies performed on a direct injection (DI) of the water samples into LC-MS/MS. The method avoids the use of additional materials and sample preparation processes, which may limit possible contamination and target compound losses (Schultz et al., 2006; Furdui et al., 2008; Dickenson and Higgins, 2013).
HPLC tubing, nylon filters, auto-sampler vial caps made of Teflon or Viton fluoropolymers, valve seals and degassers were identified as the potential sources of contamination of the instrumental blanks with PFOA (Yamashita et al., 2004; Taniyasu et al., 2005; Schultz et al., 2006; Larsen and Kaiser, 2007) and to lesser extend with PFOS (Yamashita et al., 2004). The instrumental background contamination can be reduced by replacing or bypassing the fluoropolymers parts such a degasser (Arbuckle et.al, 2013) with offline degassing of mobile phases; replacing fluoropolymer components with stainless steel, polyetheretherketone (PEEK) tubing, installing an upstream guard column, extensively flushing of the LC system or reducing the LC-column equilibration time (Martin et al., 2004; Yamashita et al., 2004; Villagrassa et al., 2006; Larsen and Kaiser, 2007; Nakayama et al., 2007; Shoemaker et al., 2009; Arbuckle et.al, 2013).
6.3 Analytical performance
The preferred analytical method for the determination of PFOS in environmental water samples, including drinking water, uses SPE followed by LC-MS/MS with electrospray ionization operating in a negative ion mode (Martin et al., 2004; Yamashita et al., 2004; Villagrassa et al., 2006; Larsen et al., 2007; van Leeuwen and Boer, 2007; Furdui, et al., 2008; Hansen et al., 2010; Sun et al., 2011; Wolf and Reagen, 2011; Post et al., 2013; Villaverde-de-Saa, 2015). Recent analytical improvements have been realized through the availability and use of high quality standards and stable isotope internal standards to compensate for the matrix effect and for inefficiencies in the extraction procedure and/or other sample preparation steps (Yamashita et al., 2004; Lowen et al., 2005; Taniyasu et al., 2005; Nakayama et al., 2007; Zainuddin et al., 2012; Villaverde-de-Saa et al., 2015). There are currently, a number of high quality analytical-grade standards that are commercially available and the list of these standards continues to expand (van Leeuwen et al., 2009, Berger et al., 2011).In the early 2000s, quantification of PFASs was biased by the lack of proper analytical standards, isotopically labelled surrogates and reference material and there was a significant analytical variability between laboratories. Two inter-laboratory studies were conducted to analyze PFASs, including PFOS and PFOA, in environmental water samples and found a varying degree of accuracy. In the first study (van Leeuwen et al., 2006), conducted in 2004/2005, factors resulting in poor agreement between participating laboratories, were determined to be low PFOA/PFOS concentrations (below 20 ng/L) in water samples; the use of low purity standards, high matrix effect, and a high background contamination in the analytical blanks. The relative standard deviation (RSD) reported in the study was 95% for PFOS (van Leeuwen et al., 2006). In the second inter-laboratory study, the performance of the participating laboratories improved due to the minimization of the matrix effects; the use of higher quality (purity and isomeric composition) shared standards (provided by a single source), and the use of mass-labelled internal standards. The reported RSD value in this study was 29% for PFOS.
Methods using SPE and DI procedures followed by LC/ESI/MS/MS have been reported in the literature for the determination of PFAAs, including PFOS in water samples (Yamashita et al., 2004; 2005; Taniyasu et al., 2005; 2013; Furdui et al., 2008; Hansen et al., 2010; Berryman et al., 2012; Zainuddin et al., 2012; Villaverde-de-Saa et al., 2015). Details regarding the preconditioning procedures of the SPE cartridges, eluent, clean-up procedures, MS quantification parameters and QC procedures specific to each method are available in the cited reference.
A study reported a limit of detection (LOD) [signal-to-noise (S/N) = 3:1] of 0.2 ng/L and a limit of quantitation (LOQ) (S/N= 10:1) of 0.66 ng/L using an SPE followed by LC-MS/MS for analyzing PFOS in surface water. A water sample of 500 mL was loaded on the Oasis WAX cartridge, a target fraction was eluted, dried under nitrogen gas and before the analysis the samples were filtered. The recovery value of 109±4% for PFOS was calculated by isotopically-labelled internal standards calibration (Sun et al., 2011; Li et al., 2011).
Villaverde-de-Saa et al. (2015), using an SPE followed by LC-MS/MS, developed a method for the determination of seven PFCAs (C6-C12) and PFOS in environmental waters samples. A water sample of 1.0 liter, fortified with internal standards, was loaded on the Oasis HLB cartridge. The method reported a LOD of 0.04 ng/L and a LOQ of 0.12 ng/L for PFOS, (LOD and LOQ were calculated as 3 and 10 times the standard deviation, respectively). The recovery value of 99±7% for PFOS was calculated by isotopically-labelled internal standards calibration.
Furdui et al. (2008) investigated the concentration of PFASs in water samples from the Great Lakes. The analysis of nine target contaminants including PFOS, were performed by directly injecting the samples into LC-MS/MS. Quantification was performed using internal standard correction and standard addition. An isotope dilution provides the most accurate and precise results. The method had a LOQ (signal-to-noise [S/N] =10:1) of 0.5 ng/L for PFOS (Furdui et al., 2008).
The province of Québec reported results of the monitoring PFASs at 16 sites, including seven drinking water treatment plants. A total of 226 water samples (84 raw, 84 treated and 58 surface water samples) were analyzed. Both raw and treated water were sampled monthly for a period of one year. Sampling of the surface water was limited through the year. The samples were analyzed using C18 cartridges and LC-MS/MS in positive ionization mode. Reported DLs ranged from 0.5 to1.0 ng/L and 0.3 to 0.6 ng/L for untreated (250 mL analysed sample) and finished water (500 mL sample), respectively. In order to compensate and correct the instrumental variations and the matrix effect, isotopically-labelled internal standards were added prior to the LC (Berryman et al., 2012). Although the photoionization technique is less sensitive than the electrospray ionization, it is less prone to matrix effect (Martin et al., 2004).
Berger et al. (2004) compared different mass spectrometric techniques (time-of-flight [TOF] high resolution MS, triple-quadrupole tandem MS, and IT-MS) coupled with a high performance liquid chromatography (HPLC) for analysis of PFASs including PFOA. The instrument parameters such as vaporizer temperature, collision energy, and cone voltage fragmentation were optimized for each mass spectrometry technique. Negative electrospray ionization was selected as the ionization mode for all instruments. The study indicated that both TOF high resolution MS and triple-quadrupole tandem MS methods had higher sensitivities than IT-MS for all tested PFASs. Although IT-MS had a higher DL and smaller linear range, it provided the best results for tentative structure elucidation and qualitative analysis of branched PFASs isomers (Berger et al., 2004; Jahnke and Berger, 2009).
The analysis of PFASs in environmental water samples has been dominated by the use of LC coupled to MS or MS/MS, although other techniques such as 19F nuclear magnetic resonance (NMR) and gas chromatography (GC)-MS have also been explored. 19F NMR analysis is a less sensitive and non-specific method due to the determination of the presence of CF2 and CF3 moiety in the sample. Gas chromatography (GC) can be used to determine neutral and volatile PFASs and fluorotelomer alcohols. PFAAs are derivatized in order to be amenable for GC analysis. However, the use of the derivatization techniques is limited for PFOS analysis due to the instability of the PFOS derivatives (Moody et al., 2001; Villagrassa et al., 2006).
7.0 Treatment technology
The available data and calculated pKa (-3.27) values indicate that PFOS is a strong acid which predominantly dissociates to a negatively charged form (anion) at environmentally relevant pH values (Brooke et al., 2004). Given the hydrophobic and oleophobic nature of the fluorinated alkyl chain and the hydrophilic nature of the sulfonic group, hydrophobic and electrostatic effects likely influence PFOS adsorption (Higgins and Luthy, 2006; Xiao et al., 2011). Due to the shorter carbon-fluorine bond length and high electronegativity of fluorine atoms in the PFOS structure, Senevirathna et al. (2010) suggested that higher adsorption of PFOS could occur on anion exchange resins. The nature of the chemical structure of PFOS (i.e., strong carbon-fluorine [C-F] bonds) makes it resistant to hydrolysis and biodegradation as well as to photolysis and several chemical treatment processes (3M, 1999; Lange et al., 2006; ATSDR, 2009).7.1 Municipal scale
Dickenson and Higgins (2013) evaluated the ability of wide range of full-scale treatment techniques to remove PFASs, including PFOS and PFOA, from raw water and potable water reuse plants. The treatment trains varied, but generally included coagulation followed by physical separation, aeration, chemical oxidation, UV irradiation, and disinfection. Regardless of the treatment train applied, there was little or no decrease in PFOS and PFOA concentrations and the authors concluded that these treatment methods are not effective in removing PFASs.GAC adsorption and membrane filtration techniques appear promising for removal of PFOS in drinking water, achieving treated water concentrations below 0.6 µg/L (Tang et al., 2006; Lampert et al., 2007; Deng et al., 2010; Takagi et al., 2011; Appleman et al., 2014). In order to achieve a PFOS concentration below 0.6 µg/L, the GAC system must be specifically designed and appropriately operated for PFOS removal in drinking water. The presence of natural organic matter (NOM) in the source water may deteriorate GAC performance by directly competing for adsorption sites and preloading (fouling) the GAC beds. Therefore, the effectiveness of GAC to remove PFOS in drinking water appears to be dependent on the regeneration frequency and/or replacement of the carbon (Kolstad 2010; Takagi et al., 2011; Appleman et al., 2014). Membrane filtration such as reverse osmosis (RO) and bench-scale nanofiltration (NF) studies demonstrated effective removal of all tested short-and long-chain PFASs including PFOS in drinking water. Although the RO process is effective, it is likely to be an expensive treatment method (Steinle-Darling et al., 2008); Quinones and Snyder, 2009; Appleman et al., 2013; Flores et al., 2013). Anion exchange resins may also be effective in removal of PFOS. However full-scale evaluation of this technology has not been conducted specifically for PFOS removal in drinking water.
The selection and effectiveness of each treatment strategy is driven by several factors, including source water chemistry, concentration of PFOS and/or other PFASs and pre-existing treatment processes. If long-chain PFASs are detected in the drinking water sources, the utility may consider the implementation of treatments such as GAC. However, utilities that have shorter chain PFASs in their raw water source may choose to implement RO. The treatment technologies need to be designed specifically for PFASs removal and operated appropriately in order to achieve contaminants removal objectives in drinking water (Dickenson and Higgins, 2013).
The ability of various drinking water treatment processes and treatment trains to remove PFOS have been summarized by Dickenson and Higgins (2013) and Rahman et al. (2014). Appendix A summarizes the percentage removal of PFOS in full-scale plants where both raw and finished water concentrations were reported (Rahman et al., 2014). Data show that the treatment technologies employed by these plants (with the exception of GAC, RO and NF) did not appreciably remove PFOS. They also show that in some cases concentrations in the finished water were higher than in the raw water, likely due to the breakdown of precursor compounds to form PFOS during the treatment (Takagi et al., 2008; Shivakoti et al., 2010). Takagi et al. (2011) also postulated that these higher finished water levels may result from desorption from GAC filters used for long periods of time without reactivation.
7.1.1 Conventional treatment
Conventional drinking water treatment processes generally incorporate coagulation, flocculation, sedimentation, and filtration, followed by primary and secondary disinfection. Common coagulants used in drinking water include aluminum sulfate (alum), ferric hydroxide, ferric chloride, polyaluminum chloride and coagulant aid polymers. Filtration media can consist of sand (single media); sand and anthracite (dual media); or sand, anthracite, and garnet (multi or mixed garnet media). GAC may also be used as the filter media.Conventional full-scale drinking water treatment techniques have been found ineffective in removing PFOS from source waters. Samples collected from several full scale conventional treatment plants indicated essentially no difference in the PFOS concentrations between plants influent and concentrations in water following the coagulation, sedimentation, and sand filtration steps (Loos et al., 2007; Shivakoti et al., 2009; Takagi et al., 2011; Thompson et al., 2011). Similarly, Eschauzier et al. (2012) reported that slow- and rapid- sand filtrations were ineffective for PFOA and PFOS removal. The inability of conventional water treatment to remove PFOS and PFOA may be due to their extremely low concentrations in water and their hydrophilicity which renders them unamenable to removal by conventional treatment processes (Rahman et al., 2014). These findings are in agreement with recently conducted bench-scale studies of the removal of PFOS from water (Deng et al., 2011; Xiao et al., 2013).
Jar tests (Xiao et al., 2013) achieved an approximately 3% removal of an influent concentration of 0.1 µg/L (100 ng/L) of PFOS, with an alum dose of 30 mg/L and pH of 7.9. A removal efficiency below 10% was reported under a range of alum doses ranging from 10 to 60 mg/L and pH levels ranging from 6.5 to 8.0. Removal rates of approximately 25% were observed using enhanced coagulation with alum doses greater than 60 mg/L and pH 4.5 - 6.5. In general, the removal efficiencies were below 35% under the examined coagulation conditions (alum doses 3-110 mg/L and pH 4.5-8.0). Ferric chloride coagulation exhibited similar results. The authors indicated that removal rates were higher for PFOS than PFOA in both conventional and enhanced coagulation conditions, possibly due to PFOS having a higher molecular size and a potential for being more hydrophobic.
7.1.2 Adsorption
Adsorbents typically used in drinking water treatment include activated carbon, resins, activated alumina, zeolites, clays, metal oxides, hydroxides, and carbonates (AWWA, 2011; U.S. EPA, 2012a). GAC is used in a fixed bed, while PAC is generally added directly to the raw water as a powder or mixed with water to form a slurry.Several laboratory studies of PFOS and PFOA adsorption kinetics indicate that PAC reached sorption equilibrium in 4 hours while GAC reached equilibrium in 168 hours, (Yu et al., 2009) and that PFASs removal percentages were generally higher for PAC than for GAC (60-90% versus 20-40%, respectively) for 10 minutes adsorption time (Hansen et al., 2010). These results may be due to PAC's smaller particle size, and higher specific surface area per volume of carbon when compared to GAC (Yu et al., 2009; Hansen et al., 2010). If PFASs are present in the raw water year round, Rahman et al. (2014) suggested that GAC adsorption may be the preferred method for PFASs removal, while PAC may be more appropriate for short-term spill response remediation.
7.1.2.1 Granular activated carbon
Full-scale evaluations of the effectiveness of GAC adsorption for the removal of PFOS in drinking water sources have been mixed. Several full-scale studies, specifically designed and operated for PFASs removal in drinking water, observed successful removal of PFOS by GAC with a long empty bed contact time (EBCT) and an appropriate regeneration regime (MDH, 2008a; Takagi et al., 2011; Appleman et al., 2014). Other water treatment plants found similar PFOS levels in both source and finished water, suggesting that GAC treatment only partially removes this contaminant, if at all. These treatment plants were not specifically designed for PFASs removal in drinking water. As the GAC had been in place for a variable period of time, it was likely that the preloading by NOM had deteriorated the GAC performance leading to similar PFOS levels in the influent and treated water (Shivakoti et al., 2010; Takagi et al., 2011; Eschauzier et al., 2012, Flores et al., 2013).A full-scale GAC treatment system with a flow rate of 1.5 m3/minute was specifically designed for PFASs removal in groundwater. The system used two GAC contactors in a lead/lag configuration with an EBCT of 13 minutes each. The lead vessel operated for approximately 18 months and treated 59,483 bed volumes (BVs) before the concentration of PFOS exceeded 0.05 µg/L. The GAC unit was capable of reducing an influent PFOS concentration in the range of 0.53-1.38 µg/L to below 0.05 µg/L, in the treated water from the lag vessel, for 72,775 BVs (approximately 22 months). At that point, the lead vessel water reached 0.11 µg/L PFOS, its carbon was replaced with virgin media and the vessel was put in the lag position (Appleman et al., 2014).
A monitoring survey conducted in UK indicated that the contaminated groundwater with an influent concentration ranging between 1.7 and 3.8 µg/L PFOS was reduced to below 0.2 µg/L using GAC treatment. The five GAC contactors were operated in a parallel-staggered mode with an EBCT of 110 minutes and a regeneration frequency of 12 months. A PFOS concentration of 0.3 µg/L (breakthrough) was found to occur between 8,000 and 9,000 BVs. However, the breakthrough concentration was reported for the period of time prior to the increase of the regeneration frequency of 24 months to 12 months (Rumsby et al., 2009).
The behaviour and fate of PFASs, including PFOS and PFOA, was assessed by analyzing influent and treated water from several drinking water treatment plants that included GAC in the treatment train. These plants were not specifically designed for PFASs removal in drinking water. The hydraulic retention time of individual treatment steps was considered when the efficiency of each these steps was assessed (Shivakoti et al., 2010; Takagi et al., 2011; Eschauzier et al., 2012; Flores et al., 2013). The studies found that only the GAC step was capable of removing PFASs in drinking water. Removal of between 63% and 97% of PFOS was reported when a GAC process was included in the treatment train (Shivakoti et al., 2010; Flores et al., 2013; Appleman et al., 2014). The paragraphs below provide more details on some of these studies.
A full-scale 5 million gallons per day (MGD) treatment plant, designed to remove trace levels (ng/L) of organic contaminants in surface water, consisted of river bank filtration, softening, UV/H2O2, biologically-active GAC filtration and six GAC contactors. The GAC system operated in parallel mode with an EBCT of 10.5 minutes. Water samples analyzed before and after the GAC system demonstrated reduction of an influent PFOS concentration of 2.3 ng/L to 0.25 ng/L (89% removal) (Appleman et al., 2014).
Eschauzier et al. (2012) monitored the concentrations of PFOS and PFOA in a drinking water treatment train consisting of coagulation, rapid sand filtration, dune passage (water slowly passed through sand dunes), softening, ozonation and GAC treatment. Only the GAC step was effective for PFASs removal. The system used two-stage GAC contactors in a lead/lag configuration. Of the 40 filters, 20 were used in parallel mode as a first stage and the other 20 were used as a second stage filter. Each GAC filter operated at a flow rate of 348 m3/hour and an EBCT of 20 minutes, resulting in a total EBCT of 40 minutes. Each virgin GAC filter was installed as a second stage filter and was switched to the first stage after 15 months of operation. After another 15 months, the carbon was reactivated and put back in service as a second stage filter. The GAC system effectively removed perfluorononanoic acid (PFNA), PFOS and perfluorohexane sulfonic acid (PFHxS). The GAC step was capable of reducing an average influent PFOS concentration of 11 ng/L in the feed water to the first GAC stage to below the LOQ of 0.23 ng/L (approximately 98 % removal) after the second stage GAC filter (Eschauzier et al. 2012). Flores et al. (2013) reported 64% removal of PFOS in a water treatment plant, which had 24 GAC contactors installed and that were regenerated approximately once a year.
Takagi et al. (2008, 2011) investigated the behaviour, fate and removal efficiency of PFOS and PFOA in drinking water treatment processes from several drinking water treatment plants that included GAC in the treatment train. The removal efficiency of PFOS and PFOA were less than 50% in many of the water treatment plants. A negative removal rate in certain plants suggested that desorption from GAC filters, used for long periods of time without reactivation, may be responsible for these observations. The negative removal rates could also result from the formation of PFOS and PFOA from the degradation of the precursor compounds found in the raw water (Takagi et al., 2011). However, PFOS was effectively removed for 8 months in a 1.5 MLD water treatment plant after the replacement of its activated carbon in the GAC unit. The treatment train consisted of a coagulation/sedimentation, rapid sand filtration and two GAC contactors (coal and coconut-shell carbon) in parallel mode. Both GAC contactors were capable of reducing the PFOS concentrations in the range of 2.3-3.9 ng/L to below the LOQ of 0.5 ng/L during the 8 month study period.
Rapid small-scale column tests (RSSCTs) are a common bench scale test used to evaluate GAC. Using RSSCTs, Appleman et al. (2013) compared the effectiveness of three different types of GAC for removal of several PFASs including PFOS and PFOA. The column experiments were conducted with an EBCT of 0.38 minutes using de-ionized water and surface water [dissolved organic carbon (DOC) of 1.7 mg/L], both spiked with 1.0 µg/L of each PFASs. The tests were run for a total of 125,000 BVs (approximately 33 days). Carbon performance varied based on the type of carbon and water chemistry, with GAC being more effective at removing PFASs in deionized water. Of the three carbons, F300 achieved the best results. In the experiments conducted with deionized water, a PFOS concentration in the filtered water was less than 0.02 µg/L (less than 2% of influent C0), after 98,000 BVs. However, the filtered water concentration reached 0.2 µg/L (20% of C0) after 11,000 BVs (3 days) in spiked surface water. Although RSSCTs are not suitable for evaluating the effect of preloading/fouling of GAC columns by DOC, the observed rapid breakthrough in the spiked natural water demonstrated that the presence of DOC affects the GAC performance in the removal of PFASs by directly competing for adsorption sites (Appleman et al., 2013).
The efficiency of PFOS removal by GAC adsorption is impacted by NOM in source water which competes for the carbon adsorption site and will adsorb irreversible, causing the carbon's capacity for the target compound to be reduced. When the adsorption capacity of the GAC is exhausted, it must be removed from the contactor and replaced with fresh or reactivated carbon. GAC is used in a fixed bed reactor, as a substitute for existing filtration media (i.e., sand) in a conventional filter, as a layer in a multi-media rapid filter, or in a separate contactor. The reactor can be located at the beginning of the treatment train in a dual-media or sand-replacement mode, or later in the treatment train as a second-stage contactor. The rate of GAC exhaustion will vary substantially for the same water source depending in which configuration GAC will be employed. A dual media (GAC and sand) is used when turbidity removal and the adsorption/removal of the contaminants are combined in a single unit process. The dual media filter (typically located after sedimentation) is likely to be exposed to higher DOC concentrations, and this filter will be exhausted faster. A GAC contactor located at the end of a treatment train will likely experience slower preloading/fouling, since the treatment steps prior to a GAC contactor will reduce the DOC influent concentrations. This treatment strategy will assist in completely utilizing the entire GAC capacity and reducing operating cost (i.e., carbon replacement cost) (Crittenden et al., 2012).
Close monitoring of PFOS breakthrough (treatment objective) is necessary for efficient operation of GAC unit. Studies indicated that PFOS was successfully removed from drinking water when the frequent regeneration or replacement of the GAC was performed (e.g., Wilhelm et al., 2008; Rumsby et al., 2009; Takagi et al., 2011). Takagi et al. (2011) observed that GAC regenerated over periods greater than one year were not effective in removing PFOS and PFOA and suggested regenerating the carbon 2 to 3 times per year. A full-scale 2,500 gpm GAC treatment plant, using two GAC contactors in series observed breakthrough of PFOA and PFOS after 286 days and 550 days, respectively. With the replacement of the GAC at the earliest time of PFOA breakthrough, the system was able to treat 1.9 million gallons of water for 23 months (MDH, 2008a; Kolstad, 2010).
Eschauzier et al. (2012) observed that the removal efficiencies of PFASs by GAC increased with increasing carbon chain length and that sulfonate compounds were removed for a longer period of time than the carboxylate compounds. Shorter-chained PFASs (especially perfluorobutanoic acid ([PFBA] and perfluorobutane sulfonate [PFBS]) were not removed by GAC. These findings were in agreement with previous batch experiments showing that the sorption of PFASs on activated carbon decreased with decreasing the carbon chain-length and perfluorosulfonates adsorbed stronger than perfluorocarboxylates with the same carbon chain length (Ochoa-Herrera and Sierra-Alvarez, 2008; Hansen et al., 2010; Dudley et al., 2012; Appleman et al., 2014). Branched isomers of PFOS and PFOA were found to be less sorbable to GAC than linear isomers. Desorption of shorter chain PFASs due to competition for sorption sites with longer chain PFASs or NOM (i.e., DOC) may result in higher levels of shorter chain PFASs in the treated water (Eschauzier et al. (2012).
7.1.2.2 Powdered activated carbon
No full-scale data were reported on the efficacy of PFOS removal by PAC. Most published studies on the efficacy of PAC were conducted at the bench-scale. PFOS concentrations in some of these bench-scale studies were order of magnitude higher than the concentration observed in natural waters. However, trends observed for PAC in terms of preferential adsorption (chain-length dependence) and competition with NOM were similar to that documented for GAC (Hansen et al., 2010; Dudley et al., 2012).Dudley et al. (2012) evaluated the adsorbability of ten PFASs with different carbon chain lengths (from C4 - C10) on commercially available PACs (coconut shell, lignite, wood, and bituminous coal) and superfine PACs (S-PACs) obtained by wet-milling the commercially obtained PACs. Sulfonate substances were found to be more adsorbable than carboxylate substances and sorption kinetics were faster with S-PACs when compared to PACs. The removal efficiencies of PFAAs increased with increasing carbon chain length (i.e., negligible removal of C4 compounds but greater than 90% removal for C7-C10 compounds). The presence of NOM was found to decrease the effectiveness of PFAS removal by PAC in batch studies. The authors also concluded that significant removal of smaller chain PFASs may not be achievable at practical PAC dosages (Dudley et al., 2012).
Yu et al. (2009) investigated the sorption kinetics and isotherms of PFOS and PFOA on PAC, GAC and an anion-exchange resin. The anion exchange resin had the highest sorption capacity for PFOA while PAC was found to be the adsorbent of choice for PFOS. Another laboratory experiment reported 97% and 24% removal of PFOS by PAC and GAC, respectively, based on an initial concentration of 1.4 µg/L in groundwater. The study also observed that PFOS sorption on PAC was faster than on GAC, suggesting that the sorption kinetics were influenced by the size of the activated carbon (Hansen et al., 2010).
7.1.3 Membrane filtration
There are four main types of membrane filtration processes in drinking water treatment applications: microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO). Low pressure membranes such as MF and UF are not capable of rejecting PFASs since their pores sizes are larger than the effective diameter of the PFASs molecules (~1 nm) (Tsai et al., 2010; Rahman et al., 2014). Bench-scale studies indicated that the membrane molecular weight cut-off (MWCO) of NF/RO is probably the most important factor for removal of PFASs for these technologies. In general, NF membranes have a lower rejection (95%) than RO (greater than 99%), which is consistent with the fact that NF membranes have larger pores (Tang et al., 2006, 2007; Steinle-Darling and Reinhard, 2008; Lipp et al., 2010; Appleman et al., 2013; Rahman et al., 2014).The available scientific information on the removal of PFOS and PFOA from drinking water supplies by membrane filtration is limited to one full-scale RO drinking water utility (Flores et al., 2013) and several indirect potable water reuse plants (Quinones and Snyder, 2009; Appleman et al., 2014). Due to the physical location of these indirect potable water reuse plants, they were considered to be major potential contributors to the drinking water facilities' source water in the conducted studies (Quinones and Snyder, 2009).
Although a full-scale conventional treatment was reported as being ineffective for PFOS removal in surface water; a greater than 99% removal of PFOS was achieved when RO followed the conventional treatment train (Flores et al., 2013). Samples were collected, taking into consideration the hydraulic retention time of each step, to assess the efficiency of each step of treatment train. The RO system's feed water was filtered by the conventional treatment process and blended with untreated groundwater. Feed water PFOS concentration ranged from 61 to 86 ng/L and the RO system was capable of reducing these concentrations to an average concentration of 0.7 ng/L (Flores et al., 2013).
Two indirect potable water reuse plants with RO units in their treatment trains were capable of reducing a PFOS concentration to below 0.25 ng/L in the RO treated water. Both RO systems had a flux rate of 12 gallons per square foot per day (gfd) (20 L/m2/h) and water recovery in the range 80-85%. The feed PFOS concentrations to the RO units ranged from 3 to 18 ng/L (Dickenson and Higgins, 2013; Appleman et al., 2014). A survey of several drinking water utilities and indirect potable water reuse plants showed that the PFOS concentrations in the treated water were comparable to the levels found in the raw water samples in almost every case. However, removal was only observed in one planned potable reuse facility when an integrated membrane treatment consisting of MF and RO was employed. The membrane system was capable of rejecting a feed PFOS concentration of 41 ng/L to below 1 ng/L (Quinones and Snyder, 2009).
Bench-scale experiments evaluated the rejection behaviour of unfouled and fouled NF membranes on the removal of PFASs, including PFOS (Appleman et al., 2013). The study found that a polyamide thin film composite flat-sheet NF membrane was capable of rejecting all of tested compounds in the range of 93 to 99%. Greater than 99% rejection of an average influent PFOS concentration of 866 ng/L (LOQ of 10 ng/L) was observed in all experiments using virgin membranes and spiked de-ionized water; virgin membranes and spiked groundwater; and fouled membranes and spiked groundwater. The fouling layer on the NF membrane showed no negative effect on PFOS rejection (Appleman et al., 2013). Another bench-scale study was conducted on one RO (MWCO of 100 Da) and three NF (MWCO range of 200 - 360 Da) membranes for the removal of PFOS in water. The RO membrane achieved a 99.9% rejection of PFOS from the feed water concentration of 2100 ng/L with a permeate PFOS concentration in the range of 2-3 ng/L. The RO system was operated with a flux rate of 30-40 L/m2/h and a feed pressure of 8 bars (116 psi). All tested NF membranes achieved a rejection in the range of 99.8-100% of an average feed PFOS concentration of 3000 ng/L, with a flux rate of up to 70 L/m2/h and an operating pressure in the range of 4-7 bars (58-101 psi) (Lipp et al., 2010).
Although there is limited information on full-scale RO and only bench-scale NF treatment information, both technologies are considered effective for PFOS removal from drinking water (Appleman et al., 2013; 2014). The results of the NF studies are promising since NF is a less energy intensive process than RO. Testing of the selected NF membrane for PFOS removal at both pilot- and full-scale is an important step for utilities when considering this treatment process. Since the size exclusion is an important mechanism for PFASs rejection by NF membranes, consideration should be taken to select membranes with MWCO smaller than the size of PFOS.
Considerations when using RO treatment include disposal of the reject water and the potential for increased corrosivity of the treated water. RO rejects a significant portion of the influent water as contaminant-rich brine, and the concentrate discharge must be disposed of appropriately. The removal of contaminants can cause mineral imbalances that could increase the corrosive nature of the treated water. In most cases, post-treatment corrosion control measures need to be taken.
7.1.4 Ion exchange
PFAAs are in an anionic form at ambient water pH values and therefore would be expected to be amenable to removal by anion exchange resins (Senevirathna, et al., 2010). Two primary mechanisms, hydrophobic and electrostatic interactions, were proposed for the removal of PFAAs by ion exchange resins (Carter et al., 2010; Deng et al., 2010; Xiao et al., 2012).Appleman et al. (2014) reported results for the only one known full scale application of ion exchange for the removal of PFASs. However, this system was not specifically designed for PFASs removal in drinking water. A 350 gpm full-scale ion exchange plant reduced the concentrations of PFOS in the range of 2.6-4.5 ng/L to below the detection level of 0.25 ng/L in groundwater. A strong base anion resin impregnated with iron oxide used for arsenic removal was assessed for PFOS removal after the resin had been in use for 5 and 9 months. The highly porous strong base anion exchange resin, achieved greater than 75% removal of PFOA, partial removal of perfluoroheptanoic acid (PFHpA) (46%), and high removal of PFOS (>92%) and perfluorohexanesulfonate (PFHxS) (97%). Shorter carbon chain compounds such as PFBA, and perfluorohexanoic acid (PFHxA) exhibited little to no removal. Results also indicated that perfluorosulfonic acids were preferably removed by anion exchange resin over the perfluorocarboxylic acids (Appleman et al., 2014).
Exchange resins exhibit a degree of selectivity for various ions, depending on the concentration of ions in solution and the type of resin selected. Laboratory-scale evaluations of different types of resins (i.e., ion exchange resins, non-ion exchange resins) for removal of PFASs in water have been reported in the literature (Lampert et al., 2007; Carter et al., 2010; Deng et al., 2010; Senevirathna et al., 2011; Xiao et al., 2012; Chularueangaksorn et al., 2013). Batch kinetic tests conducted with a high initial PFOS concentration (mg/L) demonstrated a greater than 99% removal of PFOS in 25 hours of contact time using a commercial anion exchange resin, while another anion resin achieved only 32% removal. The study also observed that PFOS anions were preferably removed over PFOA anions in the ion exchange process (Lampert et al., 2007). In laboratory experiments, anion exchange resins demonstrated high capacity for PFOS removal in water (Senevirathna, et al., 2010; Chularueangaksorn et al., 2013; 2014). Laboratory-scale fix-bed columns with an EBCT of 1.3 minutes were evaluated for PFOS removal using five commercial anion exchange resins and GAC. The breakthrough goal was set at 90% removal efficiency and the tests conducted for 122 days. Most of the anion exchange resins demonstrated a higher capacity than GAC. One resin was capable of reducing an influent PFOS concentration of 5 µg/L to 0.05 µg/L (99% removal) achieving a run length of 56,000BVs (52 days) and demonstrated adsorption capacity of 455 mg/g. GAC reached the breakthrough concentration of 0.5 µg/L faster (40 days) than the anion exchange resins (Chularueangaksorn et al., 2014). Senevirathna et al. (2010) investigated the sorption behaviour of PFOS on two ion exchange polymers, three non-ion exchange polymers and GAC. Kinetic experiments demonstrated that the ion exchange polymers and GAC were capable of reaching an equilibrium concentration after 4 hours of operation, while non-ion exchange polymers needed 10 to 90 hours. At an equilibrium concentration of 1 µg/L PFOS, the sorption capacity of the tested material decreased in the following order: ion exchange polymer > non-ion exchange polymer > GAC. However at the lower equilibrium concentration of 0.1 µg/L the non-ion exchange polymers outperformed the ion exchange polymer and GAC (Senevirathna et al., 2010).
Anion exchange resins (in chloride form) with a different polymer matrix and porosity have also been tested for PFOS removal in water (Deng et al., 2010). The study was conducted with a high initial PFOS concentration (mg/L). The experiments demonstrated that the polymer matrix was a critical factor affecting the sorption rate of PFOS. Macroporous and gel-type polyacrylic resins exhibited faster sorption and higher capacity than macroporous polystyrene resins, followed by the gel-type polystyrene resins. Since the polyacrylic matrix is more hydrophilic than the polystyrene matrix, PFOS easily diffused into the polyacrylic resin pores. Both macroporous and gel-type polyacrylic resins demonstrated a similar kinetic profile and reached an equilibrium concentration after 48 hours. However, the macroporous and gel-type polystyrene resins required greater than 168 hours to reach the sorption equilibrium. While the porosity of polyacrylic resins had little influence on the sorption kinetic and capacity, the macroporous polystyrene resins demonstrated faster sorption and higher capacity than the gel-type polystyrene resins. The low sorption of PFOS on the gel-type polystyrene resins demonstrated a size exclusion effect. Since the amount of PFOS sorbed on both polyacrylic resins was greater than the chloride anions released from the resins, the authors concluded that interaction other than anion exchange were involved in the process (Deng et al., 2010). While findings showed that anionic resins had a higher capacity for PFOS than non-ion exchange resins (Deng et al., 2010; Senevirathna, et al., 2010; Chularueangaksorn et al., 2013, 2014), it was also observed that moderately polar non-ionic resins performed better than non-polar non-ionic resins for removal of PFOS from water (Xiao et al., 2012).
Studies indicate that the ion-exchange process is a promising technology (Dickenson and Higgins, 2013) for the removal of PFAAs, including PFOS, from drinking water. However, additional studies are needed on the selectivity of the resins, kinetic limitations, the impact of DOC, regeneration rates and the presence of competing ions such as sulfate and nitrate on removal efficiency (Dickenson and Higgins, 2013; Rahman et al., 2013).
7.1.5 Oxidation, UV irradiation and advanced oxidation processes
Advanced oxidation processes (AOPs) have been developed for removal of contaminants that are resistant to more typical chemical oxidation treatment processes. They include the use of appropriate combinations of ultraviolet (UV) light, chemical oxidants and catalysts (e.g., ozone, hydrogen peroxide, titanium dioxide,) to generate highly reactive radicals, such as hydroxyl radicals, which are strong oxidants and react rapidly and non-selectively with organic contaminants.Rahman et al. (2014) summarized studies that have demonstrated that PFASs compounds like PFOS will likely be resistant to oxidation, even by molecular ozone and hydroxyl radicals. Due to the low reactivity of PFOS with ozone and AOPs, chlorine-based oxidation processes will likely not oxidize PFOS under typical drinking water conditions. The resistance of PFOS to oxidation is due to the shielding effect of the fluorine atoms and the strength of the carbon-fluorine bonds (3M Company, 1999; ATSDR, 2009).These conclusions have been confirmed in surveys of full-scale treatment plants (Quinones and Snyder, 2009; Shivakoti et al., 2010; Takagi et al., 2011; Eschauzier et al., 2012; Flores et al., 2013) described below.
In a survey of several drinking water utilities, Quinones and Snyder (2009) observed that PFOS was resistant to chlorination, chloramination and ozonation even in a combination with other treatment processes such as coagulation/flocculation, deep bed filtration and UV irradiation. Results from drinking water treatment plants in Japan reported similar concentrations of PFOS in samples of water treated with an ozonation process and in the raw water samples (Shivakoti et al., 2010; Takagi et al., 2011). Ozone doses in the range 0.37-0.85 mg/L and contact time as high as 120 minutes showed that the ozonation process was ineffective to degrade PFOS concentrations at four utilities (Takagi et al., 2011). Thompson et al. (2011) observed that an ozone dose of 5 mg/L with a contact time of 15 minutes in reclaimed water was not effective at reducing PFOS concentrations in the range of 0.9-1.4 ng/L.
In a full scale 5 MGD water treatment plant that applied a UV dose of 500 MJ/cm2 in combination with 4 mg/L of H2O2, no degradation of 11 ng/L PFOS in surface water was observed (Appleman et al., 2014). Only one utility reported a partial reduction (approximately 35 %) of an influent PFOS concentration in the range of 18-27 ng/L. The utility used two UV reactors each capable of treating 3 MGD groundwater with a UV dose of 80 mJ/cm2 and UV transmittance of 95% (Appleman et al., 2014). Although the observed partial removal of PFOS in this water treatment plant, the oxidation/AOPs appear ineffective for PFOS removal in drinking water (Appleman et al., 2014). However, ozone or AOPs may be able to oxidize polyfluorinated precursor chemicals that may be present in raw water, resulting in the potential for increasing the concentration of PFOS and PFOA in the finished water (Rahman et al., 2014).
7.1.6 Aeration/air stripping
The removal of compounds using air stripping is based on the equilibrium partitioning of chemicals between air and water, which is affected by the contact surface area between the air and the water, as well as temperature, vapour pressure, ionic strength and the pH of the water.Dickenson and Higgins (2013) evaluated 23 PFASs (including PFOS and PFOA) in raw and finished drinking water and at various steps along the treatment train. They found that aeration was ineffective at removing PFOS and PFOA (10% removal).
7.1.7 River bank filtration (soil aquifer treatment)
River bank filtration (RBF) is a drinking water treatment method where surface water flows through the subsurface sand and gravel layers of the bank or bed of a river to extraction wells and contaminants are removed through the processes of filtration, sorption, dilution and biodegradation.A drinking water utility utilizing RBF with a hydraulic residence time of approximately 10 days observed approximately 20% removal for PFOS, variable removal of some PFASs, and increases in concentration for other PFASs (Appleman et al., 2014). The authors concluded that this variation was possibly due to the variability in the influent concentrations from wastewater effluents impacted the drinking water sources and/or due to breakdown of precursor compounds through the river bank. Dickenson and Higgins (2013) concluded that RBF was not likely to result in significant removal of PFASs.
7.1.8 Emerging technologies
Other potential treatment technologies for removal of PFOS and PFOA have promise, but are still being researched actively. They have not yet all been evaluated on drinking waters by laboratory, pilot, or full-scale studies, but have been mentioned in reviews of bench scale studies of some PFASs removal from drinking water and wastewater (Vecitis et al., 2009; Eschauzier et al., 2012).Nanomaterials are being developed for drinking water treatment applications, including ion exchange, sorption and oxidation processes, and abiotic reduction (e.g., nanozerovalent iron) (Boyd et al., 2013). Different nanomaterials/nanotechnologies show promise for removal of PFOS including carbon nanotubes (CNTs), chitosan-based molecularly imprinted polymers (MIPs), electrospun nanofibrous membranes (ENFMs), and titanium dioxide (TiO2) assisted photocatalysis (Yu et al., 2008; Deng et al., 2012; Dai et al., 2013). CNTs are carbon molecules composed of carbon lattices that can take the form of tubes. Chitosan is a natural polysaccharide based on the shells of crustaceans. It may be prepared as a nanoparticle or electrospun in nanofibers (Sonia and Sharma, 2011; Zhao et al., 2011; Boyd et al., 2013). Molecular imprinting is a technique where specific sites for target compounds are constructed on a polymer so that specific adsorbates are recognized in the sorption process. ENFMs are prepared by electrospinning nanofibers of polymer or polymer composite materials to create membranes of non-woven fibers with diameters ranging from several hundreds to tens of nanometers (Greiner and Wendorff, 2007; Dai et al., 2013; Boyd et al., 2013).
Deng et al. (2012) have demonstrated the effectiveness of CNTs for removal of perfluorinated contaminants, including PFOS, from aquatic environments when the hydrophobic interactions were involved in the sorption of perfluorinated contaminants onto the CNTs. The sorption of the perfluorinated contaminants was increased with increasing carbon-fluorine chain length with the same functional group. Yu et al. (2008) investigated a chitosan-based molecularly imprinted polymer (MIP) for sorption of PFOS and concluded that the MIP sorbent had a high sorption capacity and selectivity for PFOS and it could have application in both water and waste water treatment for removal of PFOS. Electrostatic interaction played an important role in the adsorption process.
Dai et al. (2013) investigated the morphology, structure and physicochemical properties of the multi-walled carbon nanotube (MWCNT)-filled electrospun nanofibrous membranes (MWCNT-ENFMs) for sorption of PFOS. The authors concluded that these membranes showed promise as sorbents for removal of PFOS from aqueous solutions. The sorption kinetics and isotherm results showed faster sorption rates and higher sorption capacity onto the MWCNT‑ENFMs than onto the pure ENFMs for PFOS removal. The authors also concluded that it would be possible to further optimize the MWCNT-ENFMs to increase their sorption capacities while also preventing the release of the MWCNTs into the water (Dai et al., 2013).
7.2 Residential scale
Generally, it is not recommended that drinking water treatment devices be used to provide additional treatment to municipally treated water. In cases where an individual household obtains its drinking water from a private well, a private residential drinking water treatment device may be an option for reducing PFOS concentrations in drinking water.Although there are no certified residential treatment devices for the reduction of PFOS from drinking water, available data suggests that residential activated carbon and reverse osmosis can achieve treated PFOS concentrations of 0.2 µg/L and below 0.05 µg/L, respectively. In addition, treatment devices using anion exchange may be effective for the reduction of PFOS.
The Minnesota Department of Health (MDH, 2008b) completed a study of the effectiveness of point-of-use (POU) water treatment devices for PFOA, PFOS and PFBA removal and demonstrated that RO and activated carbon filters were capable of reducing PFOS concentrations typically found in drinking water. Laboratory screening tests and field evaluation of devices installed in municipal water systems were undertaken. In the laboratory tests, the challenge waters had a PFOS concentration of 3.0 µg/L and the target goals for performance was reduction to 0.2 µg/L. The field testing involved monitoring and sampling of four activated carbon and seven RO point-of-use devices installed at two municipal wells. One of the wells had concentrations of 0.6 µg/L PFOA, 0.9 µg/L PFOS, and 1.4 µg/L PFBA, while only PFBA was present (1.5 µg/L) at the second well. All RO devices were equipped with an activated carbon pre-filter (before the RO membrane) and a post-treatment (after the RO membrane) activated carbon polishing filter. Results indicated that all activated carbon and RO devices were effective in removing PFOS to below the quantification limit of 0.2 µg/L and below the detection limit of 0.05 µg/L, respectively. Based on these results, activated carbon and RO are expected to be effective at reducing the level of PFOS in drinking water to levels below the proposed MAC of 0.6 µg/L.
Health Canada does not recommend specific brands of drinking water treatment devices, but it strongly recommends that consumers use devices that have been certified by an accredited certification body as meeting the appropriate NSF International (NSF)/American National Standards Institute (ANSI) drinking water treatment unit standards. These standards have been designed to safeguard drinking water by helping to ensure the material safety and performance of products that come into contact with drinking water. Certification organizations provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). In Canada, the following organizations have been accredited by the SCC to certify drinking water devices and materials as meeting NSF/ANSI standards (SCC, 2015):
- CSA Group (www.csagroup.org);
- NSF International (www.nsf.org);
- Water Quality Association (www.wqa.org);
- UL LLC (www.ul.com);
- Bureau de normalisation du Québec (www.bnq.qc.ca); and
- International Association of Plumbing & Mechanical Officials (www.iapmo.org).
Activated carbon filtration systems may be installed at the faucet (POU) or at the location where water enters the home [point-of-entry (POE)]. RO systems are intended for POU installation, as larger quantities of influent (incoming) water are needed to obtain the required volume of treated water, which is generally not practical for residential-scale point-of-entry systems. RO systems should only be installed at POU as the water they have treated may be corrosive to internal plumbing components. A consumer may need to pre-treat the influent water to reduce fouling and extend the service life of the membrane.
Ion exchange treatment technology using anion exchange resins may also be a feasible for PFOS removal in residential scale applications. Ion exchange treatment devices is typically designed and constructed for residential use by drinking water treatment system providers or dealer. Health Canada strongly recommends that homeowners ensure that these systems are constructed using materials certified to NSF/ANSI Standard 61 (NSF/ANSI, 2014). If an ion exchange system is used, the water may need to be filtered through a GAC filter to remove any chlorine or chloramine (if connected to a treated water supply) from the water before it reaches the resin.
Before a treatment device is installed, the water should be tested to determine general water chemistry and verify the presence and concentration of PFOS. Periodic testing by an accredited laboratory should be conducted on both the water entering the treatment device and the finished water to verify that the treatment device is effective. Devices can lose removal capacity through use and time and need to be maintained and/or replaced.
8.0 Kinetics and metabolism
PFOS is considered chemically unreactive, and it is not metabolized. The oral absorption of PFOS is rapid and complete (Kemper, 2003; Hundley et al., 2006; Lau et al., 2007). Once absorbed, PFOS is primarily restricted to plasma and extracellular fluid (Chang et al., 2012) and excreted in urine.8.1 Absorption
PFOS is rapidly and nearly completely absorbed through the GI tract. In rats, studies consistently estimated the oral absorption rates of PFOS at >95% after a single dose (4.2 to 20 mg/kg) by gavage (Johnson and Ober, 1979; 1999; Cui et al., 2010).No controlled studies investigating the oral availability of PFOS have been conducted in humans, but there is evidence of oral absorption of PFOA from studies of residents living in areas with contaminated drinking water (Emmett et al., 2006; Hölzer et al., 2008; PFOS was not investigated in these studies). Emmett et al. (2006) correlated the number of glasses of tap water ingested per day with blood concentrations of PFOA, indicating this was the primary exposure route. Hölzer et al. (2008) correlated the litres of drinking water consumed per day with serum levels of PFOA; although there was no relationship noted with drinking water consumption and PFOS levels in either study, the main exposures in these communities were to PFOA.
No inhalation or dermal studies have reported PFOS kinetics; however, the physicochemical properties of the compound suggest that these routes of exposure are not important when PFOS is found in drinking water (see Section 5.7).
8.2 Distribution
The highest PFOS concentrations in rats were measured in the liver after a single oral gavage dose of 400 mg/kg (Benskin et al., 2009) and 2 weeks of dietary exposure to 400 mg/kg bw per day (De Silva et al., 2009). This might be due to the binding of PFOS to liver proteins in rat, including the liver fatty acid binding protein (L-FABP) (Luebeker et al., 2002). Similarly, the highest PFOS concentrations were found in liver and serum after intraperitoneal injection of PFOS in female rats (1 or 10 mg/kg bw per day for 2 weeks) (Austin et al., 2003). PFOS was also accumulated primarily in mouse livers after dietary exposure to 0.031 or 23 mg/kg bw per day for 1-5 days (Bogdanska et al., 2011) or a single oral dose of 1 or 20 mg/kg bw (Chang et al., 2012), with liver-to-blood ratios ranging from 2 to 6 (Bogdanska et al., 2011). One of the oral dosing mouse studies also identified storage of PFOS in lungs (lung-to-blood ratios of 1.5-2; Bogdanska et al., 2011). Average liver-to-serum PFOS concentration ratios in monkeys ranged from 0.9 to 2.7, after oral bolus administration of 0.03, 0.15, or 0.75 mg/kg bw per day for 183 days (Seacat et al., 2002). The average percent PFOS dose found in the livers of these monkeys ranged from 4.4 ± 1.6% to 8.7 ± 1.0%.Few data have been gathered on the human tissues to which PFOS is typically distributed. In tissues taken from human cadavers in the U.S., the mean liver-to-serum concentration ratio was 1.3, suggesting there is no extensive binding to liver protein in humans as measured in the rat (Olsen et al., 2003a). Maestri et al. (2006) measured a lung to blood ratio of 1.5 from pooled human samples. Neither cerebrospinal fluid (Harada et al., 2007) nor thyroid (Pirali et al., 2009) have been observed to be relevant partitioning sites for PFOS.
PFOS, as with other perfluoroalkyls, binds to serum albumin and, to a lesser extent, to plasma γ-globulin, α-globulin, α-2-macroglobulin, transferrin and β-lipoproteins (ATSDR, 2009; Butenhoff et al., 2012a). Binding of PFOS to the plasma lipoprotein-containing fractions appears to be limited (≤ 9%) in human, as shown in vitro with the plasma of a human donor (Butenhoff et al., 2012a). PFOS has also been shown to competitively bind to the human thyroid hormone transport protein transthyretin (TTR), with less than one-tenth of the T4 affinity (Weiss et al., 2009).
Sex and age have been demonstrated to influence PFOS distribution in rodents and humans. Sex-related differences were observed in maximal serum concentrations of PFOS measured in Sprague-Dawley rats (single oral dose of 2 mg/kg) with females having a 2.5 times higher level than males. At 15 mg/kg, however, the differences were not seen. Chang et al. (2012) also reported slightly slower elimination for PFOS in female rats. After dietary exposure ranging from 4 to 14 weeks (0.5, 2.0, 5.0 and 20 ppm in food), PFOS levels increased proportionally with cumulative dose in both sexes (Seacat et al., 2003). Average serum levels in females were approximately 31-42% higher than in males with liver concentrations reported as being equal. Liu et al. (2011) reported varying distributions of PFOS with age in mice administered a single subcutaneous dose (50 mg/kg) on postnatal day (PND) 7, 14, 21, 28 or 35. The levels of PFOS in the liver increased with age at exposure, whereas the PFOS levels in brain decreased with levels at PND 7 two times that at PND 35. In contrast, the blood PFOS levels did not vary with postnatal age. As previously described in Section 5.6, serum PFOS levels in humans appeared to be influenced by age and gender in CHMS. The effect was also observed in U.S. studies. In the general U.S. population (NHANES data for 1999-2008), the serum PFOS levels were reported to be significantly higher in males than in females, regardless of age. As age increased, PFOS concentration increased in both sexes and the increase was more pronounced in females than in males (Kato et al., 2011). PFOS blood levels were shown to be influenced by age and gender, with lower PFOS serum in females than in males in the age group 20-50 years; a plausible explanation is menstrual bleeding, as well as gestation and lactation transfer (Harada et al., 2004; Ingelido et al., 2010). The analysis of mother-children data indicated that PFOS levels were on average 42% higher in children than in their mothers, and that this trend persisted until at least 19 years of age. The PFOS child:mother ratios were higher in boys aged 5 years than in girls (Mondal et al., 2012).
PFOS exposure can occur transplacentally and lactationally. Fetal and pup PFOS concentrations in serum and brain were higher than corresponding maternal concentrations in rats exposed to 0.1-1.0 mg/kg bw per day from gestational day (GD) 20 to PND 21 (Chang et al., 2009). Serum concentrations in newborn rats exposed in utero to 1-10 mg/kg bw per day on GD 2-21 mirrored the maternal administered dose and were similar to those in the maternal circulation at GD 21 (Lau et al., 2003). Serum PFOS concentration in mouse pups administered a daily oral bolus from GD 1 to 17 (1, 5, 10, 15 or 20 mg/kg/day) were consistent with the rat data (Thibodeaux et al., 2003). In humans, PFOS cord blood levels have been shown to correlate with maternal serum concentrations (Inoue et al., 2004; Midasch et al., 2007; Needham et al., 2011; Gützkow et al., 2012a); moreover, maternal serum PFOS concentrations have been observed to decrease throughout pregnancy (Fei et al. 2007). PFOS has been measured in breastmilk samples collected worldwide (So et al., 2006; Kärrman et al., 2010; Roosens et al., 2010; Kadar et al., 2011; Sundström et al., 2011). A correlation has been reported for PFOS levels in human milk and maternal serum with average milk to maternal serum concentration ratios ranging from of 0.01 to 0.03 (Liu et al., 2011). PFOS breastmilk concentrations were shown to decrease as the number of infants breast-fed by a mother increased (Tao et al., 2008; Kadar et al., 2011). Most studies reported decreases in PFOS breastmilk (Thomsen et al., 2010) or maternal serum (von Ehrenstein et al., 2009; Monroy et al., 2008) throughout the lactation period; however, Tao et al. (2008) reported an upward trend for PFOS levels in milk through 6 months of lactation.
8.3 Metabolism
The available data indicate that PFOS is not metabolized. Based on the available evidence for PFOA, metabolism is not expected to play a role in the clearance of PFOS (Kemper and Nabb, 2005; EFSA, 2008; ATSDR, 2009).8.4 Excretion
Remarkable species-dependent differences in elimination half-life have been observed, with PFOS remaining in human bodies for a much longer duration than in other species, including non-human primates, rats and mice. Species- and sex-related differences are primarily attributed to elimination kinetics where, at higher doses, the kinetics of PFOS in rodents and primates do not follow one-compartment or simple first-order models (Andersen et al., 2006). The arithmetic mean half-life value for serum elimination of PFOS in humans was calculated as 5.4 years (95% CI = 3.9-6.9 years; range = 2.4-21.7 years) based on data obtained in retired workers (Olsen et al. 2007); no half-life could be found from general population studies. The half-life of PFOS in animals varies depending on experimental protocols, including the duration animals are followed, but is on the order of days to weeks in rodents and months in monkeys (see Table 1).| Species | Dosing regime | Mean half-life (days) | Reference |
|---|---|---|---|
| Rat | Single oral dose of 2 mg/kg-bw; followed for 24 hours | 3.10Footnote a (M) 1.94 ± 0.13Footnote b (F) |
Chang et al., 2012 |
| Single oral dose of 4.2 mg/kg-bw; followed for 144 hours | 8.23Footnote a (M) | ||
| Single i.v. dose of 2 mg/kg-bw; followed for 24 hours | 7.99 ± 4.94Footnote b (M) 5.62Footnote a (F) |
||
| Single oral dose of 2 mg/kg-bw; followed for ≥10 weeks | 38.31 ± 2.32Footnote b (M) 62.30 ± 2.09Footnote b (F) |
||
| Mouse | Single oral dose of 1 mg/kg-bw; followed for 20 weeks | 42.81Footnote c (M) 37.80Footnote c (F) |
Chang et al., 2012 |
| Single oral dose of 20 mg/kg-bw; followed for 20 weeks | 36.42Footnote c (M) 30.45Footnote c (F) |
||
| Monkey | Single i.v. dose of 2 mg/kg-bw; followed for up to 161 days | 132 ± 7Footnote b (M) 110 ± 15Footnote b (F) |
Chang et al., 2012 |
| Oral dose of 0.15 or 0.75 mg/kg-bw per day for 182 days | ~200Footnote c (M & F) | Seacat et al., 2002 | |
| Human | 26 former workers with an average of 31 years of work and 2.6 years retired; external exposure data not provided | 1971 (1424-2517)Footnote d | Olsen et al., 2007 |
In humans, the loss of blood during menstruation may contribute significantly to excretion in women (Harada et al., 2005). Lactation can also be a significant route of excretion in women (von Ehrenstein, 2009; Kim et al., 2011a).
8.5 Physiologically-based pharmacokinetic (PBPK) models
Several models with varying complexities have been developed to describe the kinetics of PFOS in both experimental animals and humans (Andersen et al., 2006; Tan et al., 2008; Loccisano et al., 2011; 2012a; 2012b; 2013). Due to the non-linear nature of PFOS pharmacokinetics, where faster clearance is seen with high bolus dosing, physiological models can provide an improved means of assessing cross-route and cross-species dosimetry for risk assessment.The first model developed for PFOS was a biologically-motivated compartmental pharmacokinetic (PK) model for monkeys, which included saturable renal resorption of filtered PFOS (Andersen et al., 2006). Subsequent work to refine the model included the addition of a liver compartment and of time-dependent functions for protein binding and volume of distribution to fit high-dose monkey and rat oral and intravenous plasma, urine and feces kinetic data (Tan et al., 2008). PFOS PBPK models for adult rats (Loccisano et al., 2012a), monkeys (Loccisano et al., 2011) and humans (Loccisano et al., 2011) were built upon the compartmental models; however, the time-dependency function for volume of distribution was removed. Further models for lactation and pregnancy were developed for rats (Loccisano et al., 2012b) and humans (Loccisano et al., 2013). No models have been developed for mice, and no pregnancy and lactation models have been developed for monkeys. The basic structure of the PBPK model was the same for all three species, with only time-dependent changes in physiology included to describe pregnancy and lactation along with the time-dependency for plasma and tissue binding. The models included tissue compartments for gut (for oral/dietary dosing), skin (human and monkey model only; for dermal dosing), liver, fat, and kidney, with remaining body tissues grouped together (and not divided into richly and poorly perfused compartments). Biliary excretion and fecal elimination of the unabsorbed bolus oral dose or dietary exposure was added to the rat model; moreover, the rat version did not include a fat compartment (which became lumped with the rest of the body) or physiological gut, which was described as a one compartment non-physiological compartment. The PBPK model assumes only the plasma free fraction of PFOS is available for uptake into tissue, excretion or resorption. Elimination from plasma is described as glomerular filtration of the free fraction into a filtrate compartment. The filtered PFOS can either be eliminated in urine or resorbed into the kidney where it can return to systemic circulation. Finally, the rat PFOS model included protein binding in the liver, which was described as being saturable. The models were relatively good at reproducing controlled dosing data for rats (dietary, oral gavage, and IV routes of exposure; Loccisano et al., 2012a), and monkeys (IV and oral gavage routes of exposure; Loccisano et al., 2011). Although no controlled dosing data were available for humans, biomonitoring data (for typically only a single timepoint) were within similar ranges as model simulations (using measured water concentrations for the biomonitored populations, along with assumptions on ingestion patterns; Loccisano et al., 2011).
The Loccisano models for humans (2011), monkeys (2011) and rats (2012a) were considered for use in the current assessment (see Section 10); moreover, a mouse model (that was scaled from the rat model, but that could not be validated) was used. Further specifics of the models used for this assessment, including the values used for each of the physiological and chemical-specific parameters, are described in Campbell and Clewell (2013). Exposure was described as a constant intake of PFOS in humans (ingesting 1.5 L of water per day) and the model was allowed to reach steady state conditions prior to determining the predicted drinking water concentration consistent with the internal dose metric. The human was simulated as a 70 kg adult.
The Andersen et al. (2006) pharmacokinetic model was modified by Wambaugh and colleagues (2013) by adding a gut compartment for oral absorption and specifying an upper limit on tissue distribution. The authors used the model to translate dose regimes and available LOEL, NOEL, and benchmark dose (BMD) values from 13 in vivo studies of PFOS into internal dose metrics (area under the curve, average, and maximum serum concentrations). The data were modelled for cynomolgus monkeys, Sprague-Dawley rats, and CD-1 mice. A Bayesian approach was employed to model ranges of various physiological parameters. Wambaugh et al. (2013) identified relatively good concordance between predicted and measured (at study termination) serum concentrations, with few outliers, and identified that no single dose metric appeared to be best for all adverse endpoints. Dose metrics for points-of-departure (PODs) tended to be similar (with the exception of immune studies, which had lower PODs than other endpoints), indicating consistency between species and most adverse outcomes.
Dermal and inhalation routes from contact with drinking water were not included in as potential routes of exposure in this effort, as their contribution to exposure is considered to be negligible (see Section 5.7).
8.6 Animal-to-human extrapolation
Although animal-to-human extrapolations are typically discussed after the selection of potential PODs, consideration has been given to this extrapolation earlier in the PFOS assessment, as the large variability between species can affect POD selection. The large differences in PFOS clearance between humans and other species must be accounted for when using animal studies as a basis for human risk assessments. The application of default approaches for animal-to-human extrapolation-such as the use of an interspecies uncertainty factor of 10 or allometric scaling-might not be sufficiently protective of humans, who receive longer internal exposures to target tissues. For this reason, chemical-specific approaches that can account for pharmacokinetic differences between species and nonlinear behavior of PFOS were considered for the risk assessment. These approaches include the application of chemical-specific adjustment factors (CSAFs) and PBPK modelling. A discussion and application of each of these approaches is outlined below, and further details can be found in a report prepared for Health Canada by Summit Toxicology (2015).8.6.1 Derivation of CSAFs
A major advantage of the application of CSAFs over default uncertainty or allometric scaling factors is that the approach incorporates both species- and chemical-specific data. Despite this strength, the approach relies on single values representative of pharmacokinetics in species, and does not necessarily account for non-linear pharmacokinetics.IPCS guidelines on calculating CSAFs (IPCS, 2005) were applied to derive the toxicokinetic portion of the interspecies uncertainty factor (AKUF). IPCS recommends that the default interspecies uncertainty factor of 10 be divided into values of 4.0 (100.6) for the toxicokinetic portion (AKUF) and 2.5 (100.4) for the toxicodynamic component (ADUF). The default AKUF of 4.0 becomes replaced with any AKUF values calculated based on chemical-specific data (IPCS, 2005). As data were not available to quantitatively evaluate toxicodynamic differences between species, no ADUF was calculated.
To calculate the AKUF (i.e., reflecting interspecies toxicokinetic differences), the following equation was used:
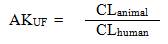
Figure 1 - Text Equivalent
- AKUF is the toxicokinetic component of the interspecies uncertainty factor; and
- CL is clearance in animals and humans (e.g. mL/kg bw per day).
Clearance was not measured directly in humans, and must be calculated based on half-life estimates using the following equation:
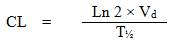
Figure 2 - Text Equivalent
- CL is clearance in animals and humans (L/kg bw per day);
- ln 2 is the natural log of 2;
- Vd is the volume of distribution, which is the theoretical volume of blood in which the amount of a chemical would need to be uniformly distributed to produce the observed blood concentration; and
- T½ is the half-life of a compound.
8.6.2 PBPK modelling
A typical approach for PBPK modelling is to use the model to calculate human-relevant PODs, which are derived by applying a human PBPK model to internal dose metrics (e.g., concentrations of PFOS in plasma) that were either calculated or measured in animals. With sufficiently validated models, this approach is considered to be the most robust approach for performing animal-to-human extrapolations. However, there is only medium confidence in human, monkey, and rat models, because different model codes were used for different species, and model fits to some datasets were not optimal. Moreover, the limited understanding of reasons for the observed sex differences in clearance in rats means there are weaknesses in how this could be addressed in the model. Finally, a major drawback in using the standard PBPK modelling approach is that human models have not been fully verified. Human data available for verification are limited to biomonitoring studies that allow for only rough estimates of exposure scenarios, and for which serum concentration measurements were typically only performed once (with a few populations with measurements at two timepoints). Loccisano and colleagues did not develop PBPK models for mice, but Health Canada modelling approaches using mouse studies scaled the rat models using mouse data. As insufficient toxicokinetic data exist to verify whether the mouse model is appropriate, confidence in the mouse model is low. Therefore, there is insufficient confidence to use precise PBPK model results as PODs for the risk assessments.As an alternative approach to using the PBPK model for POD calculations, ratios of PBPK model-predicted dose metrics were used to calculate AKUF values for relevant doses. This approach is thought to provide more robust estimates of the AKUF than the traditional calculations described in Section 8.6.1, as it can address the non-linear kinetics of PFOS, identifying different values at steady state for different oral dose levels. In contrast, the AKUF values calculated above are dependent on the specific doses and dose regimes used in the pharmacokinetic studies; uncertainties arise in the values obtained from these studies, as the animals were provided only single doses, and the human data were not obtained from controlled dosing studies. Furthermore, clearance-based AKUFs are ratios of low doses in humans to high doses in animals, and therefore exposures between the species are not of the same magnitude; using the PBPK model to derive the AKUF allows for a more appropriate comparison of doses of the same magnitude.
The selected dose metric for the PBPK-derived AKUF values was steady-state concentrations of PFOS. Steady-state concentration was selected as it is typically relevant for chemicals with long half-lives, and is a conservative assumption. Although alternative dose metrics-including peak concentrations (Haber et al., 2013)-might be more predictive of PFOS toxicity for certain adverse endpoints, additional work was not performed to further investigate the most appropriate dose metric. Plasma was selected as the relevant tissue for steady-state concentrations, as it is a metric that can act as a relevant proxy for a wide variety of organs, because blood flows to these different organs. Liver steady state concentrations were also incorporated into the assessment for comparison with plasma-based AKUF values, as the liver has been identified as a primary organ for PFOS distribution in pharmacokinetic studies, and is also a potential target organ for toxicity. However, there is lower confidence in liver-based values than plasma values, as very little pharmacokinetic data exists to be able to perform verification of the PBPK model for liver concentrations. Liver concentrations could not be verified for humans, mice, and monkeys; for rats, minimal verification could be performed, but as data were available for only one to two timepoints in each study, the comparisons are not robust. Moreover, the use of plasma concentrations as a proxy for a variety of organs simplifies the application of AKUF values in the assessment, which is already complex due to the use of AKUF values that are species- and dose-specific.
Using the Loccisano PBPK models described in Section 8.5, steady-state plasma and liver concentrations were calculated at various doses in each of the species. The same doses were used for each of the species. For each dose run in the PBPK model, ratios of steady-state PFOS concentrations in humans vs. other species were calculated to obtain dose- and species-specific AKUF values (Summit Toxicology, 2015). Steady-state concentrations and AKUF values for plasma and liver at potentially relevant doses are listed in Table 2.
| Metric | Species | Oral dose (mg/kg bw per day) | |||
|---|---|---|---|---|---|
| 0.001 | 0.01 | 0.1 | 1 | ||
| Steady-state plasma PFOS predictions (µg/mL) | Human | 5.40 | 53.0 | 360 | 530 |
| Monkey | 2.85 | 26.6 | 140 | 239 | |
| Mouse | NCFootnote a | NC | 17.1 | 170 | |
| Rat | 0.349 | 3.69 | 36.9 | 368 | |
| Steady-state liver PFOS predictions (µg/mL) | Human | 20.1 | 197 | 1340 | 1977 |
| Monkey | 10.6 | 98.8 | 521 | 894 | |
| Mouse | NC | NC | NC | NC | |
| Rat | 4.45 | 20.7 | 195 | 1935 | |
| AKUF derived based on plasma predictions | MonkeyFootnote b | 2 | 2 | 3 | 2 |
| MouseFootnote c | NC | NC | 21 | 3 | |
| Rat | 16 | 14 | 10 | 1 | |
| AKUF derived based on liver predictions | MonkeyFootnote b | 2 | 2 | 3 | 2 |
| MouseFootnote c | NC | NC | NC | NC | |
| Rat | 5 | 10 | 7 | 1 | |
8.6.3 Recommended interspecies extrapolation approach
The recommended approach for interspecies extrapolation is the use of a PBPK model for the calculation of the AKUF component of the CSAF, using steady-state plasma concentrations as the dose metric. The use of plasma concentrations ensures the relevance of the dose metric to adverse effects that occur in a variety of organs. Organ-specific dose metrics are typically preferred over blood-based values, whenever available; however, using the plasma metrics for this assessment provides consistency in the application of the AKUF over a wide variety of adverse endpoints. AKUF values were calculated for liver metrics for comparison with plasma values; the behaviour of PFOS in the liver was similar to that in the plasma, and AKUF estimates were on the same order in both compartments. These results indicate that plasma values are appropriate proxies to be used for adverse hepatic effects. The liver-based values in rats were slightly lower than plasma-based values; however, greater confidence is placed in the plasma values because more data were available to verify this compartment of the PBPK model. The dose- and species-specific AKUF values (for steady-state plasma concentrations) in Table 3 are applied in Sections 10.1 and 10.2.Although PBPK-derived AKUF values were selected as the recommended approach for this assessment, several weaknesses have been identified. As described above, the AKUF was plasma based rather than being organ specific. Steady state concentrations were also selected as the dose metric, as a conservative assumption relevant to the nature of the compound; detailed work was not performed to identify whether other dose metrics (e.g. peak concentrations) would be more appropriate for the various adverse endpoints. Steady state was also not reached in the human model at doses below 0.1 mg/kg bw per day. Furthermore, PBPK models have not been developed specifically for the mouse, and have been scaled instead from the rat, without further pharmacokinetic data for mice to be used for verification; the application of AKUF values for rats is therefore recommended for use in mice in the absence of robust data in the species. AKUF values for rats are also derived based on male rats only, despite sex differences in the species.
Despite these weaknesses, using the PBPK model to derive the AKUF was thought to be of equal or greater robustness to other potential interspecies extrapolation approaches. The selected approach quantitatively incorporates pharmacokinetic differences among species, in a manner that addresses the non-linear kinetics of PFOS, which cannot be done using the default AKUF-derivation approach. Moreover, the PBPK-derived AKUF values do not rely on individual pharmacokinetic studies that are often single-dose studies and not easily comparable among species. An ideal approach to address species differences would be to use blood concentrations of PFOS-either by using pharmacokinetic models to estimate the concentrations, as employed by Wambaugh et al. (2013), or using the values specifically measured in individual studies-as PODs for the assessment. However, the human PBPK models that would be used to extrapolate from this serum concentration cannot be fully verified based on existing human pharmacokinetic data, which decreases the level of comfort of using this approach to estimate precise PODs. The recommended approach was selected as a means of quantifying interspecies differences while addressing the non-linear kinetics of PFOS, without relying on precise PBPK-derived estimates of PODs.
9.0 Health effects
Many studies have been conducted to investigate the effects of PFOS on health. The summary of literature on health effects for PFOS is largely based on a comprehensive review conducted by a consultant (Sanexen Environmental Services Inc., 2013), and includes only the studies of direct relevance to the derivation of the health-based value.9.1 Effects in humans
9.1.1 Acute toxicity
No epidemiological data regarding acute or short-term toxicity of PFOS were located.9.1.2 Subchronic and chronic toxicity
Many quality epidemiological studies have been conducted. Large cohorts of workers and environmentally exposed populations have been followed, with observations of significant relationships between exposure to PFOS and lipid levels, liver and thyroid functions, and reproductive (fecundity, age of puberty, and sperm quality), immunological, and developmental (birth weight) outcomes. Although all of these studies present limitations to some extent, including in terms of study design, bias and confounders, the human weight of evidence provides a strong argument in favor of detrimental health effects of the compound. This information should support the choice of a health endpoint; however, deriving a safe exposure dose based on studies in humans remains a challenge because of the difficulty in characterizing a dose-response pattern with current studies. Their use in the present assessment is important to verify the relevance of animal to human extrapolation, and the monitoring of future studies will help in determining the accuracy of the observed associations.Most environmental studies among PFAA-exposed populations were conducted in the Mid-Ohio Valley within the C8 Science Project. The C8 Science Panel was convened as a result of a class action settlement against DuPont, and is composed of independent epidemiologists jointly selected by lawyers for the community and DuPont. The C8 Health Project is the largest study of a population exposed to PFAAs in drinking water, containing residents of Ohio and West Virginia communities surrounding the DuPont Washington Works plant. The health survey was conducted in 2005-2006 on approximately 69,000 individuals, including children and adults. The main PFAA exposures in the community were to PFOA-the median PFOS serum concentrations in this population were 20.2 ng/mL, compared to 17.5 ng/mL in the general American population during the same period (Frisbee et al., 2009). Some longitudinal/prospective studies were also conducted among this population after a follow-up period. Some recent data in the project have not yet been published in peer reviewed literature; summaries of these studies-as well as panel conclusions and further information on the members of the panel-are available on the C8 Science Panel website (www.c8sciencepanel.org/panel.html).
9.1.2.1 Liver effects
Some level of association between exposure to PFOS and alteration in liver enzymes has been observed, but no clear trend has been defined. A cross-sectional study found no association between PFOS serum levels (range = 20-2,110 ng/mL) of 3M Cottage Grove employees (Minnesota) participating in the medical surveillance exam (70% of them eligible for the study) and hepatic parameters (data were not shown in the report; Olsen et al., 2003b). In another cross-sectional study, a small linear association between levels of PFOS and of Alanine transaminase (ALT) was reported in participants of the C8 project (Gallo et al., 2012). However, the clinical significance of the low magnitude in ALT increase is unknown. An occupational study compared hepatic enzymes before and after the demolition of manufacturing facilities. A significant association was found between PFOS and decreased ALT among workers with baseline PFOS levels similar to the general population. No association was found between PFOS and total bilirubin, AP or AST (Olsen et al., 2012). Overall, no definitive conclusion on liver toxicity can be drawn due to study limitations and low magnitude of enzymatic changes.9.1.2.2 Immune suppression
Studies in environmentally-exposed populations have identified associations between PFOS levels and decreased antibodies against various illnesses, but the influence of PFOS exposure on clinical immunosuppression (i.e., incidence of illnesses) appears to be more tenuous. A study in children found an inverse relationship in immune response with PFAA exposure (Grandjean et al., 2012; Grandjean and Budtz-Jørgensen, 2013), with maternal cord PFOS levels negatively correlated with anti-diphtheria antibody concentration at 5 years. Moreover, children in this population demonstrated increased odds of not reaching protective antibody levels for diphtheria after vaccination at 7 years old (Grandjean et al., 2012). The prospective nature, sample size, low risk of selection bias and defined objectives make the results relevant to the studied population; however, relevance to other populations is questionable, as increased exposure to other potential immunosuppressants occurring in this region (Faroe Islands) was not accounted for in the study. Increased PFOS exposure was also associated with decreased antibodies against rubella in children from a prospective birth cohort of pregnant women from Norway (2007-2008; Granum et al., 2013). In contrast, prenatal exposure to PFOS was not associated with hospitalizations for infections in a Danish Cohort (1996-2002; Fei et al., 2010a), nor with episodes of common cold, gastroenteritis, eczema or asthma in the aforementioned Norwegian cohort (Granum et al., 2013). In a Taiwanese cohort study, the median serum PFOS concentration was significantly higher in asthmatic children (Dong et al., 2013), and prenatal exposure to PFOS was positively correlated with cord blood IgE levels, particularly in male children; however, there was no association with atopic dermatitis (Wang et al., 2011b). Cord blood IgE levels, food allergy, eczema, wheezing, or otitis media were not associated with maternal PFOS in female infants in a prospective cohort study of pregnant women from 2002 to 2005 in Japan (Okada et al., 2012).Although some effects on the antibody response have been observed, conflicting results were common in the dataset, which remains relatively small. A low level of consistency was observed across studies, with variations between genders, specific microbial immunoglobins, infections, mother vs. child exposure, and child years, amongst other characteristics. Moreover, the risk of residual confounding, bias, and chance cannot be discarded. These flaws impede concluding on a causative mechanism, and the nature of the association remains unclear.
9.1.2.3 Lipidemia
Significant associations between PFOS and increased total cholesterol and alteration of other lipid parameters have been reported. A cross-sectional study found no association between PFOS serum levels (range 20-2,110 ng/mL) of 3M Cottage Grove employees (Minnesota) participating in the medical surveillance exam (70% of them eligible for the study) and lipid parameters (data were not shown in the report) (Olsen et al., 2003b). Careful interpretation is required because the data for these results were not published. A longitudinal study conducted in workers involved in the demolition of perfluoroalkyl manufacturing facilities found no clear association between PFOS levels and serum lipids, although some level of association was found between PFOS and increased HDL (Olsen et al., 2012). Some limitations include the self‑reporting of employees' characteristics, low participation rate, the possibility of exposure to other contaminants, and relatively short follow-up times. In contrast, an unpublished cross-sectional study found lower HDL values in male employees with the highest serum PFOS levels. Another longitudinal study conducted in 560 adults (2005-2006 with follow-up in 2010) found a decrease in LDL and total cholesterol with decreased serum PFOS level (no changes for HDL or TG) (Fitz‑Simon et al., 2013). The clinical significance is uncertain, given the low number of participants changing from the high to the normal level of cholesterol categories, the unknown mechanism of action, and the low magnitude of the changes. Cross-sectional studies within the C8 Health Project found increasing trends for total cholesterol, LDL, triglycerides, and the total cholesterol (TC)/HDL ratio with increasing PFOS (Steenland et al., 2009; Frisbee et al., 2010). A cross-sectional study of the general U.S. population indicated that adults in the highest serum PFOS quartile had higher TC levels than those in the lowest quartile (no association for serum LDL) (Nelson et al., 2010). In another cross-sectional study conducted in Inuits from Nunavik, a negative association was found between plasma PFOS level and triglycerides and the total cholesterol/HDL ratio, and a positive association was found with HDL levels (Château-Degat et al., 2010). The clinical relevance of very small alterations in cholesterol levels is unclear. Increased PFOS exposure also resulted in increased uric acid in two occupational and one general population studies (reviewed by Steenland et al., 2010). Overall, associations between PFOS and alterations in lipid parameters have been observed; however, the conclusions are limited by the lack of consistency across studies, the study designs, the possibility of selection bias, and chance finding from the high number of testing conducted.9.1.2.4 Thyroid disruption
Inconsistent effects on thyroid hormone levels were observed in PFOS-exposed populations. A cross-sectional study found no association between PFOS serum levels (range 20-2,110 ng/mL) of 3M Cottage Grove employees (Minnesota) participating in the medical surveillance exam (70% of them eligible for the study) and thyroid parameters (data were not shown in the report) (Olsen et al., 2003b). A cross-sectional study of the general population (C8 Health Project) indicated that PFOS was associated with an increase of serum total T4 (TT4) and a decrease of T3 uptake in both genders (Knox et al., 2011a). The association with PFOS and serum TT4 and T3 uptake was stronger in women, but lower than men for serum albumin. Another cross-sectional study conducted in the children enrolled in the C8 Health Project indicated a positive association between serum PFOS levels and increased TT4 levels (Lopez‑Espinosa et al., 2012). Serum PFOS levels were not associated with prevalence of thyroid disease in a cross-sectional analysis of the general U.S. population (Melzer et al., 2010). However, men from the highest serum PFOS quartile were more likely to report current (treated) thyroid disease. A cross-sectional study conducted in the Inuit population of Nunavik found negative associations between serum PFOS and serum TSH, T3, and thyroxin-binding globulin, and a positive association between serum PFOS and serum free T4 (fT4) (Dallaire et al., 2009). A matched-case control study found no association between environmental exposure to PFOS and hypothyroidism (TSH and fT4 levels) in pregnant women from Edmonton, Canada (Chan et al., 2011). A negative correlation was found between PFOS levels in fetal and maternal serum and T3 in the general population in South Korea (Kim et al., 2011a). No association was found between PFOS and TSH in a small population of anglers in New York (Bloom et al., 2010).Although associations between serum PFOS and TT4, fT4, T3 and TSH were observed, no clear trend for thyroid hormone changes related to PFOS exposure can be established because results were equivocal, it was not possible to calculate cumulative exposure, individuals with thyroid diseases were excluded, possibly biasing the results, and temporality cannot be established with the cross-sectional study design.
9.1.2.5 Kidney effects
An increased risk of chronic kidney disease (reduced estimated glomerular filtration rate) was reported in a cross-sectional study of the general U.S. population (Shankar et al., 2011). Causality will be difficult to be established for adverse kidney effects, as altered kidney function could cause an increase in serum PFOS levels.9.1.3 Carcinogenicity
Some associations between PFOS and risk of cancer of the bladder, breast, male reproductive organs, and overall cancers were observed; however, the evidence does not support the carcinogenicity of PFOS. In an occupational study, PFAA workers (n = 2,083) were found to have an elevated risk for bladder cancer mortality in the City of Decatur, Alabama (Alexander et al., 2003). However, the authors reported that it was difficult to draw any definite conclusions because there were only 3 cases of bladder cancer and the workers were exposed to several compounds concurrently (all 3 cases were production workers considered at high PFOS exposure, and had also worked at the plant incinerator or wastewater treatment plant). Moreover, no adjustment was conducted for race, smoking or other contaminants; the exposure was based on job categories; the cases with bladder cancer were not the ones with the highest exposure; and there was a risk of selection bias, lowering the credibility of the results. In a follow-up study, there was no association between PFOS exposure and an increased risk of bladder cancer (Alexander and Olsen, 2007). In an earlier analysis of the same cohort, the risks for episodes of medical care for overall and male reproductive cancers were greatest in the group of employees with the highest and longest exposures to fluorochemicals (Olsen et al., 2001). A prospective cohort study was conducted in the general population of Denmark to investigate the possible association between PFOS exposure and cancer risk (Eriksen et al., 2009). No significant correlation was found between PFOS serum concentrations and the incidence of prostate, bladder, pancreas and liver cancer across quartiles of serum PFOS. Evidence of a relationship between PFOS and breast cancer was found in a small case-control study conducted in Greenlandic Inuit women. However, considering that the risk of breast cancer was increased in relation with several chemicals, confounding by other compounds or chemical groups may have played a role in the observed association (Bonefeld-Jorgensen et al., 2011).Although some evidence of an association between PFOS and the risk of cancer has been observed, the effects were equivocal, and no clear trend could be determined due to limitations in the studies (small number of cases, confounding, and participant selection bias).
9.1.4 Developmental and reproductive toxicity
Recent epidemiological studies have observed effects on birth weight, developmental milestones, thyroid hormones, immune system, fecundity, and age of puberty, indicating that the fetus, neonate and young children may be considered as vulnerable sub-populations to prenatal and early life PFOS exposure (Apelberg et al., 2007; Stein et al., 2009; Washino et al., 2009; Andersen et al., 2010; Hoffman et al., 2010; Gump et al., 2011; Stein and Savitz, 2011; Maisonet et al., 2012 ).9.1.4.1 Developmental toxicity
Inverse associations between PFOS at early pregnancy and birth weight have been reported in different general population studies. A cross-sectional study conducted by the C8 Science Panel found an association between serum PFOS levels and low birth weight at PFOS levels above the median (Stein et al., 2009). Lower birth weight and higher weight at 20 months were observed in girls born from mothers with higher prenatal concentration of PFOS (who were previously selected for a nested case-control study of pubertal development among mothers enrolled in the Avon Longitudinal study of Parents and Children in Great Britain [ALSPAC]) (Maisonet et al., 2012). However, the results were of poor precision (-140 g, 95% CI: -238 to -42). Also, the analysis of a prospective cohort study in Japan (n = 230) indicated that birth weight of female neonates was negatively correlated with prenatal exposure to PFOS after adjustment for multiple covariates (Washino et al., 2009). Again, the results were of poor precision due to high variability in the dataset (infants were estimated to be 269 g lighter, with 95% CI ranging from 73 to 466), low participation rate (29%), and the possibility of measurement errors (as suggested by the authors). Small but significant negative associations were observed with head circumference, ponderal index, and birth weight in a cross-sectional study of the general population in Baltimore, Maryland (Apelberg et al., 2007). In contrast, no association between maternal levels of PFOS and any of the measured fetal growth indicators (placental weight, birth length and head and abdominal circumferences) was found in a random sample of women and their offspring in the Danish National Birth Cohort (Fei et al., 2008b). Other general population studies in Canada and Denmark found no associations between maternal serum PFOS levels and birth weight (Fei et al., 2007; Monroy et al., 2008; Hamm et al., 2010). PFAAs (including PFOS) were not associated with BMI and waist circumference in a prospective cohort study with long-term follow-up (20 years) among pregnant women recruited within the Danish National Birth Cohort and their male or female children ( Halldorsson et al., 2012).PFOS-induced developmental health effects on thyroid and neurobehaviour have also been investigated. A negative correlation between maternal serum PFOS levels and fetal T3 levels was found in a small South Korean cross-sectional study (but not with TSH and TT4 levels or with birth weight) (Kim et al., 2011a). In the U.S. general population, a higher risk of parental report of diagnosis of attention deficit/hyperactivity disorder (ADHD) with higher levels of PFOS was found in children aged 12-15 years (Hoffman et al., 2010). In a cross-sectional study conducted within the C8 Health Project, no association was found between PFOS and the prevalence of parents who reported that a doctor diagnosed their child with ADHD or a teacher told them their child had a learning disorder (Stein and Savitz, 2011). No association was found between maternal serum PFOS and motor or developmental milestones (fine/gross motor, attention, cognition and language) in children aged 18 months (Fei et al., 2008a), or behavioral or motor coordination problems in children aged 7 years old (Fei and Olsen, 2011).
Although some effects on development have been observed in population studies, the evidence supporting a link between early-life exposure to PFOS and developmental toxicity is equivocal because most studies were not designed to allow causal inference. The most preoccupying evidence come from the prospective studies showing an increased risk of altered birth weight in Britain and Japan; however, the clinical significance of these findings is unclear, and other larger studies would be needed to support the results considering the poor precision of the point estimate, the relatively small size of the studies and the risk of confounding and bias.
9.1.4.2 Reproductive toxicity
The main findings suggest a possible link between PFOS exposure and reduced fecundity in cohort and case-control studies and delayed puberty; however, the quality of the evidence is limited and not sufficient to define the nature of the relationship.A delay in the median age of puberty in both sexes was associated with PFOS concentrations in a cross-sectional study of individuals aged 8-18 years within the C8 Health Project (Lopez-Espinosa et al., 2011). The authors questioned the clinical significance of the results because the median age at puberty in this study was similar to the median age reported in the general U.S. population (12.5 vs 12.9 years), and the mechanisms behind the delayed onset of puberty are unclear. PFOS exposure in utero was found to be slightly (but not significantly) associated with an increased odds of earlier puberty in girls participating in the aforementioned ALSPAC cohort (Christensen et al., 2011). The authors mentioned the results could be biased by the misclassification of exposure and selection of participants.
The influence of PFOS exposures on sperm parameter observations is inconsistent. Serum levels of PFOS were associated with a decreased number of normal sperm in young Danish men aged 18.2-25.2 years in a cross-sectional study (Joensen et al., 2009). A tendency toward reduced levels of all semen parameters (testosterone, free androgen, etc.) was also found in the highest quartile. However, there was a high risk of selection bias and chance findings. A positive association between serum PFOS and a lower proportion of morphologically normal sperm of pregnant partners was identified in the combined groups of three populations from Greenland, Poland, and Ukraine (Toft et al., 2012). A few significant associations with sperm concentration and total sperm count were found in the analyses based on single countries; however, there was no overall dose-response, the results are subject to the ecological fallacy, and the authors mentioned the results were likely due to chance findings. In a cross-sectional study conducted in men from Durham, NC, a positive correlation between plasma PFOS and serum LH levels was found, but there was no association with altered semen quality (Raymer et al., 2012). Maternal PFOS at pregnancy week 30 (in utero exposure) was not associated with sperm quality and serum reproductive hormones in male offspring at age 19-21 years in a pregnancy cohort in Denmark (Vested et al., 2013). In conclusion, no clear pattern of association between maternal PFOS levels and sperm quality can be established because of studies' inconsistencies, design limitations, and high risk of selection bias.
An association between PFOS exposure and a reduction in fecundity has been observed; however, the studies were not robust and the results are inconclusive. A reduction in fecundity (increased time to pregnancy and irregular menstrual periods) was found to be associated with the plasma PFOS levels in 1,240 parous/nulliparous women from the Danish National Birth Cohort; however, the information for multiple confounders was omitted from the analysis (sperm quality, frequency of intercourse, etc.) (Fei et al., 2009). Increased relative odds of subfecundity (time to pregnancy greater than 12 months) was also reported in a case-control study on parous women enrolled in the Norwegian Mother and Child Cohort Study (no association in nulliparous women) (Whitworth et al., 2012). The lack of adjustment for confounders generates doubt on the validity of their observation. No association between serum PFOS and time to pregnancy or fecundity was found in nulliparous women in a longitudinal cohort study in Denmark (Vestergaard et al., 2012). In an Italian case-control study, couples affected by infertility tend to have higher PFOS levels and to have a higher gene expression of nuclear receptors involved in steroid and xenobiotics metabolism; however, the mechanism of action remains unclear (La Rocca et al., 2012).
The occurrence of self-reported preeclampsia in the Mid-Ohio Valley was associated with PFOS for exposure above the median; however, the results were of poor precision (Stein et al., 2009). A significant inverse association was found between PFOS and serum estradiol levels in perimenopausal and menopausal age groups of women from the C8 Health Project; however, temporality cannot be established with the study design (Knox et al., 2011b).
A prospective cohort study recruited 1,400 pregnant women (randomly out of 43,045) within the Danish National Birth Cohort (DNBC, 1988-1989) and measured PFOS concentration in their plasma (Fei et al., 2010b). Duration of breastfeeding was reported 6 and 18 months after birth by phone interviews. The risk of breastfeeding for a shorter period was higher with increasing plasma PFOS concentrations. For example, the risk (adjusted hazard ratio) of shorter breastfeeding duration (weeks) for women with plasma PFOS > 43.3 ng/mL was 1.4 (95% CI 1.2-1.6) times higher than for those with plasma PFOS 6.4-26.6 ng/mL, after adjusting for maternal age at delivery, pre-pregnancy BMI, maternal socioeconomic status, alcohol consumption and smoking (the trend for an increase in risk with increasing four-quartile comparison was also significant). Also, the odds (adjusted odds ratio) of weaning before 6 months of age was 1.20 (95% CI: 1.1-1.4) times higher for each 10 ng/mL increase in plasma PFOS when restricting the model to multiparous women (not significant in primiparous women), after statistical adjustments. A similar association was observed with weaning before 3 months of age. However, more studies would be needed to support these results, since PFOS plasma concentration was measured only one time, only 18% of eligible women participated in the DNBC study, there is a risk of outcome recall bias (mothers might not report accurately the date of weaning), and the authors did not rule out the possibility that reverse causation could explain the association (considering that women that have breastfed longer can be more likely to breastfed longer their next infants, and that PFOS is excreted in breast-milk, lowering the plasma concentration).
Correlations and associations have been observed between PFOS and altered birth weight, fecundity, fertility, sperm quality, preeclampsia, shorter duration of breastfeeding, and thyroid hormones. However, the evidence remains insufficient to clarify the nature of the relationship due to the lack of consistency across studies, important limitations in study design, and risk of bias and confounding.
9.2 Effects on experimental animals
The vast majority of animal studies stated that PFOS exposure was performed using the potassium salt of PFOS (K+PFOS), with the exception of studies by Qazi et al., which used the tetraethylammonium salt (Qazi et al., 2010b) or tetrabutylammonium salt (Qazi et al., 2009b, 2010a) of PFOS. Studies that did not state the specific salt used in their study were assumed to have used the potassium salt, as this was the most common compound used. Most of the studies did not state whether the administered dose referred to the K+PFOS compound, or specifically to the PFOS ion; only one study (Peden-Adams et al., 2008) stated that the doses reflect the concentration of PFOS ion, separate from the potassium salt. The summaries described herein use the concentrations and doses stated by authors. This approach is also used for quantitative assessments; however, as the PFOS ion contributes to 93% of the molar weight of K+PFOS and is released from the compound upon exposure, only minor quantitative differences would result from using K+PFOS and PFOS doses interchangeably.9.2.1 Acute toxicity
A mean oral LD50 value of 251 mg/kg bw was calculated for male and female CD rats based on a single administration of PFOS (100-1,000 mg/kg bw) by gavage (5/sex/group) (Dean and Jessup, 1978). An inhalation LC50 of 5,200 mg/m3 was determined in Sprague-Dawley rats (5/sex/group) exposed to PFOS dust in air (1,890-45,970 mg/m3) for one hour (Bio/Dynamics, 1979; Rusch, 1979).Single oral exposure of rodents (rats and mice) to PFOS at ≥ 250 mg/kg bw was shown to cause tonic convulsions when ultrasonic stimulus was applied to the animals (Sato et al., 2009). PFOS alone did not cause neurotoxic symptoms, morphological changes or physiological alteration (hormone concentration). Although the convulsive effect was observed at very high doses, it is considered as neurotoxicity induced by PFOS because the same ultrasonic stimulus did not cause convulsions in control animals or in animals treated with PFOA.
Skin irritation was not observed in albino New Zealand White rabbits dermally exposed to PFOS (Biesemeier and Harris, 1974). Severe eye irritation was reported in rabbits (0.1 mL ocular application, washout after 5 or 30 seconds) (Riker Laboratories Inc., 1981). Additional studies reported mild to moderate eye irritation after ocular PFOS exposure (Biesemeier and Harris, 1974; Warf Institute Inc., 1975; Hazleton Laboratories America Inc., 1987; Hazleton Wisconsin Inc., 1994; Corning Hazleton Inc., 1997).
Acute developmental neurobehaviour studies are discussed in Section 9.2.5.
9.2.2 Short-term exposure
Studies documenting the toxicity of PFOS after short-term oral exposure identified four main targets, namely the immune system, the liver, serum lipids and thyroid. The immune system appears to be the most sensitive target, with a LOAEL of 0.00166 mg/kg bw per day and a NOAEL of 0.000166 mg/kg bw per day in mice (Peden-Adams et al., 2008). The lowest LOAELs for hepatic, lipid, and thyroid effects were 0.024 mg/kg bw per day (Butenhoff et al., 2012b), 0.03 mg/kg bw per day (Seacat et al., 2002), and 0.15 mg/kg bw per day (Seacat et al., 2002), respectively. This section will focus primarily on these key effects observed at the lowest levels, and will only briefly discuss other types of changes observed in animals.9.2.2.1 Immune system effects
Immune system effects observed at the lowest levels tend to indicate that immunosuppression is the effect of greatest concern. Studies designed to identify the effects of PFOS on the immune system measured mortality from infection, changes in levels of immunoglobulins and cytokines, activity levels of immune cells, and lymphocyte phenotype and proliferation. In studies that were not designed to specifically study immunological effects (i.e. higher dose bioassays), more general immune system toxicity was measured as decreases in white blood cell counts, and organ weight and histological changes in the spleen and thymus. The various immune system effects were reported only in mice and rats, with no immunotoxicity studies designed for other species.The IPCS (2012) has presented a continuum of the strength of evidence provided for various types of data that could suggest the occurrence of immunosuppression. In animal studies, host resistance data and immune function data (including antibody production and NK cell function) provide the strongest weight of evidence for immunotoxicity. Data from observational immune assays (including lymphocyte phenotype and proliferation, and changes in cytokine levels), as well as evidence of changes in haematology and organ histopathology and weight, are all classified as providing equivocal evidence of immunosuppression. This section will discuss the effects in order from strongest to weakest weight of evidence for immunosuppression. Because several studies for PFOS provide evidence of decreased host resistance and immune function, these studies will form the greater focus of this section.
Only one study investigated the effect of PFOS on host resistance (i.e. the top tier in the IPCS framework) to infections. In the study, female B6C3F1 mice were exposed to PFOS at gavage doses of 0, 0.005 or 0.025 mg/kg bw per day for 21 days, and subsequently innoculated with Influenza A virus (Guruge et al., 2009). Increased mortality from Influenza A infection was observed in mice exposed to 0.025 mg/kg bw per day. Although B6C3F1 appears to be a strain of mice that is sensitive to PFOS effects, female mice have been demonstrated to be less sensitive than males to other immune outcomes arising from PFOS exposure.
The most sensitive effect observed in animal studies in the IPCS category of immune function data was the suppression of T-dependent antigen response (TDAR) for IgM, using sheep red blood cell (SRBC) as an antigen. The lowest LOAEL on this effect was 0.00166 mg/kg bw per day (NOAEL of 0.000166 mg/kg bw per day; Peden-Adams et al., 2008), and decreases tended to have clear dose dependence in most studies. The effect was observed at the lowest levels in adult mice in three studies described below:
- At ≥0.00166 mg/kg bw per day in male and ≥0.0166 mg/kg bw per day in female B6C3F1 mice (n = 5/dose) exposed through oral gavage (doses of K+PFOS: 0, 0.000166, 0.00166, 0.00331, 0.0166, 0.0331 or 0.166 mg/kg bw per day) for 28 days (Peden-Adams et al., 2008).
- At ≥0.083 mg/kg bw per day in male C57BL/6 mice (n = 10/dose) exposed by oral gavage (to doses of 0, 0.00833, 0.0833, 0.417, 0.833, and 2.083 mg/kg bw per day) for 60 days (Dong et al., 2009).
- At ≥0.083 mg/kg bw per day in male C57BL/6 mice (n = 6/dose) exposed by oral gavage (to doses of 0, 0.00833, 0.0167, 0.0833, 0.417, and 0.833 mg/kg bw per day) for 60 days (Dong et al., 2011).
In contrast, a study exposing male C57BL/6 mice to 0.25 mg/kg bw per day (as the tetraethylammonium salt of PFOS; no other dose levels were used in the study) for 28 days did not find any change in serum levels of anti-SRBC or anti-TNP-LPS IgM, nor in the number of splenic cells secreting anti-SRBC IgM (Qazi et al., 2010b).
PFOS-induced changes in serum levels of other immunoglobulins-which also fall under the IPCS category of immune function data-were observed at higher exposure levels. In contrast with IgM levels, IgG and IgE levels tended to be increased after PFOS exposure. In male C57BL/6 mice, increased SRBC-specific IgE and IgG were observed at the highest dose (0.833 mg/kg bw per day; see more detailed study description above) (Dong et al., 2011). Increases in non-specific serum total IgG were observed at 5 mg/kg bw per day-but not 20 mg/kg bw per day-in male C57BL/6 mice exposed to PFOS for 7 days (Zheng et al., 2011). A study of male rats exposed to 0.14-6.34 mg/kg bw per day (Lefebvre et al., 2008) explored the effects of PFOS on various IgG subtypes; the study found a significant trend for increased total serum IgG2a and IgG2c and secondary T-dependent IgG response (using KLH as an antigen). Total serum IgG1 was decreased in male rats exposed only to the two lowest doses (0.14 and 1.33 mg/kg bw per day).
The final effect in the IPCS category of immune function data was altered splenic natural killer (NK) cell activity, which was observed to be altered in mice in studies previously described above; activity tended to be increased at the lowest doses and decreased at higher doses. Decreased splenic NK cell activity was observed in male B6C3F1 mice exposed to 0.0166-0.166 mg/kg bw per day (Peden-Adams et al., 2008). In Dong et al. (2009), non-monotonic changes in the activity were observed in male C57BL/6 mice, with increases at 0.083 mg/kg bw per day, no effects at 0.417 mg/kg bw per day, and decreases at 0.833 and 2.083 mg/kg bw per day. Splenic NK cell activity was decreased in male mice exposed prenatally to ≥1 mg/kg bw per day (Keil et al., 2008) and adult male mice exposed to ≥20 mg/kg bw per day for 7 days (Zheng et al., 2009). In female mice, no changes in activity were observed at doses up to 0.166 mg/kg bw per day (Peden-Adams et al., 2008), but decreased NK cell activity was observed in those exposed prenatally to 5 mg/kg bw per day (Keil et al., 2008).
Additional immunological effects observed in studies of PFOS exposure were described by IPCS as types of data that provide only equivocal evidence of immunosuppression. Although the data support the PFOS-induced immune suppression described above, they are described only briefly as they are not robust enough to be used as a basis for a drinking water quality guideline. The observed effects were as follows (with IPCS classifications indicated in brackets):
- Alterations in subpopulations of B-cell, T-cell, and presenting antigen cells in the spleen and thymus in male and female mice, with a lowest LOAEL of 0.00331 mg/kg bw per day (Keil et al., 2008; Peden-Adams et al., 2008; Dong et al., 2009; Qazi et al., 2009b; Zheng et al., 2009). No effects on blood lymphocyte phenotype were observed in rats exposed to ≤7.58 mg/kg bw per day (Lefebvre et al., 2008) [observational immune assays].
- Alterations in levels of various cytokines in male and female mice, with a lowest LOAEL of 0.0031 mg/kg bw per day (Qazi et al., 2010a; Dong et al., 2011, 2012b; Fair et al., 2011; Mollenhauer et al., 2011; Zheng et al., 2011). The nature of effects was described by Zheng et al. (2011) and Dong et al. (2011) as appearing to create an excessive Type 1 response and deficient Type 2 response (i.e. a predominance in humoral immunity and deficiency in cell-mediated immunity, which can lead to a decreased ability to fight intracellular pathogens and cancerous cells [Guruge et al., 2009; Zheng et al., 2011]) [observational immune assays].
- Decreased cellularity and lymphocyte proliferation in male mice, with a lowest LOAEL of 0.417 mg/kg bw per day (Dong et al., 2009, 2012b; Qazi et al., 2009b) [observational immune assays].
- Reduced leukocyte counts in rats at ≥6 mg/kg bw per day (Goldenthal et al., 1978a) [haematological data].
- Evidence of increased apoptosis in spleen and thymus, at ≥0.0833 mg/kg bw per day in mice (Wang et al., 2011b; Dong et al., 2012a; Zhang et al., 2013) and ≥3.21 mg/kg bw per day in rats (Lefebvre et al., 2008) [histopathological data].
- Histological effects in thymus and spleen at ≥5 mg/kg bw per day in mice (Qazi et al., 2009b; Wang et al., 2011b; Zhang et al., 2013) and ≥18 mg/kg bw per day in rats (Goldenthal et al., 1978a; Cui et al., 2009) [histopathological data].
- Decreased absolute and/or relative weight of thymus and spleen at ≥0.417 mg/kg bw per day in male mice (Dong et al., 2009, 2012a; Qazi et al., 2009b; Zheng et al., 2009, 2011) and at 0.984 mg/kg bw per day in male rats (Butenhoff et al., 2012b) [organ weight data].
- Increased serum corticosterone in male mice at 0.25 mg/kg bw per day (Qazi et al., 2010b) and ≥20 mg/kg bw per day (Zheng et al., 2009, 2011), but not at ≤0.833 mg/kg bw per day (Dong et al., 2011) [not classified by IPCS, but supportive data].
9.2.2.2 Hepatic effects
The hepatic effects occurring at the lowest levels in short-term studies were increases in liver weight. Histological changes in the liver and increases in serum enzymes that are indicators of adverse hepatic effects were also observed at higher levels.Increased liver weight (absolute or relative) was observed in studies of varying durations, with lowest LOAELs at:
- 0.0833 mg/kg bw per day in C57BL/6 mice (10/dose) exposed via gavage for 60 days to 0.00833, 0.0833, 0.417, 0.833, and 2.083 mg/kg bw per day (Dong et al., 2009). The effect was also observed at higher doses in many other mouse studies (Thibodeaux et al., 2003; Yahia et al, 2008; Era et al., 2009; Qazi et al., 2009b, 2010a, 2010b; Zheng et al., 2009, 2011; Dong et al., 2011, 2012a; Wan et al., 2011; Wang et al,. 2011b; Zhang et al., 2013). Conversely, no increase in liver weight was observed in mice exposed up to 0.166 mg/kg bw per day for 28 days (Fair et al., 2011), 10 mg/kg bw per day for 7 days (Wan et al., 2011), or 10.5 mg/kg bw per day for 4 days (Abbott et al., 2009);
- 0.15 mg/kg bw per day in female and 1.33 mg/kg bw per day in male Sprague-Dawley rats (15/group) with exposure to 2, 20, 50, or 100 ppm PFOS in feed (0.14, 1.33, 3.21, and 6.34 mg/kg bw per day in males and 0.15, 1.43, 3.73, and 7.58 mg/kg bw per day in females) for 28 days (Lefebvre et al., 2008). The effect was also observed at higher doses in many other rat studies (Goldenthal et al., 1978a; NOTOX, 1999; Seacat et al., 2003; Thibodeaux et al., 2003; Cui et al., 2009; Yu et al., 2009a; Elcombe et al., 2012a);
- 0.75 mg/kg bw per day in monkeys (n=6) with exposure to 0.03, 0.15, and 0.75 mg/kg bw per day by oral bolus dose (Seacat et al., 2002).
Increases in histological effects were observed in short-term studies. The study in which the effects were observed at the lowest levels (Seacat et al., 2003) was of rats in 4- and 14-week early sacrifice groups of a 2-year dietary study (Butenhoff et al., 2012b; histological effects in the liver in this study are summarized in Section 9.2.3). The LOAELs in the 4-week study were 0.37 mg/kg bw per day in males and 1.77 mg/kg bw per day in females; in the 14-week study, the values were 0.34 mg/kg bw per day in males and 1.56 mg/kg bw per day in females. Hepatic hypertrophy and cytoplasmic vacuolation were observed in these dose groups. Similar effects were observed at higher doses in other rat studies (Elcombe et al., 2012a; Goldenthal et al., 1978a; NOTOX, 1999; Cui et al., 2009), and in studies of monkeys (Seacat et al., 2002). Additional hepatic gross and histological effects observed at higher doses included:
- Brown liver in male rats exposed to 3.2 mg/kg bw per day (Christian et al., 1999);
- Fatty changes in male rats exposed to ≥5 mg/kg bw per day (Kim et al., 2011b); and
- Focal or flakelike necrosis at ≥5 mg/kg bw per day and focal hemorrhage, erythrocytic transudation, and focal hepatocytic degeneration accompanied by inflammatory cellular infiltration at 20 mg/kg bw per day in male rats (Cui et al., 2009).
9.2.2.3 Serum lipid effects
Decreased total cholesterol and HDL cholesterol were the serum lipid effects observed at the lowest levels in short-term studies; the various serum lipid measurements were decreased in monkeys, mice, and rats in the vast majority of studies that considered these endpoints. Decreased LDL and triglycerides were also measured in various studies. The lowest LOAEL for this endpoint was 0.03 mg/kg bw per day.The LOAEL of 0.03 mg/kg bw per day for this endpoint was observed in a longer duration (26 week) study conducted in male and female Cynomolgus monkeys (4-6 animals per group) administered PFOS (0, 0.03, 0.15, or 0.75 mg/kg bw per day) by oral intubation of PFOS in a capsule (Seacat et al., 2002). Serum concentrations of cholesterol and triglycerides were measured before treatment and at several time points during treatment (Day 37, 62, 91, 153 and 182); HDL cholesterol was only analyzed on days 153 and 182. The changes considered consistent and statistically/biologically significant by the authors were decreased total cholesterol in both sexes at 0.75 mg/kg bw per day and decreased HDL (at 0.03 and 0.75 mg/kg bw per day in males, 0.15 and 0.75 mg/kg bw per day in females). At various time points following treatment at the lowest dose level (0.03 mg/kg bw per day), cholesterol levels were statistically significantly decreased compared to controls in male and female monkeys, and HDL levels were decreased in male monkeys, with no clear dose or time relationship. Lower levels of HDL (females) were observed at 0.15 mg/kg bw per day. Based on their statistical analyses, the investigators concluded that the NOAEL in this study was 0.15 mg/kg bw per day (LOAEL of 0.75 mg/kg bw per day) (Seacat et al., 2002).
However, both EFSA and Health Canada proposed different interpretations of the findings by Seacat et al. (2002). EFSA (2008) considered that the changes in HDL observed at this dose level were treatment-related and therefore concluded that it was justified to consider 0.03 mg/kg bw per day as a NOAEL (LOAEL of 0.15 mg/kg bw per day). Health Canada (2013c) considered that the statistical approach used in the original study (Seacat et al., 2002) was inadequate to interpret measures carried out repeatedly throughout the study and instead used linear mixed models to assess the effects of dose on these endpoints (TG, HDL, and cholesterol). Based on these models, Health Canada assessed the effect of dose and days on each endpoint and found a significant effect (p = 0.0003 to p < 0.0001) of dose on cholesterol and HDL in both sexes. There was no dose effect on the TG endpoint among both males and females, although when one outlier point for TG in males was removed, an overall significance between dose groups was observed (p = 0.0213). Differences between days were generally observed in all endpoints in both males and females. Results from Dunnett's pair wise test comparing treatment groups to control group indicate a statistically significant difference at ≥0.03 mg/kg bw per day for HDL in males, at ≥0.15 mg/kg bw per day for decreased cholesterol (females) and at 0.75 mg/kg bw per day for decreased cholesterol in males (for TG, only the lowest group was different from control when the outsider data point was removed). The time-dose interaction was significant for cholesterol in females (Health Canada, 2013c). Based on this statistical analysis, the LOAEL should be 0.03 mg/kg bw per day for decreased HDL in males (no NOAEL) and 0.15 mg/kg bw per day for decreased total cholesterol (NOAEL: 0.03 mg/kg bw per day).
The other monkey study for PFOS (Goldenthal et al., 1978b) also observed a significant reduction in serum cholesterol, at 4.5 mg/kg bw per day after 90 days of exposure. In a lower dose group (1.5 mg/kg bw per day), one of the female monkeys (i.e., half of the females in the dose group) had very low serum cholesterol.
Decreases in serum lipid parameters were also observed in other species. The LOAEL in mice was 0.166 mg/kg bw per day for total cholesterol in animals exposed for 28 days, with a NOAEL of 0.0331 mg/kg bw per day (Fair et al., 2011). Decreased triglycerides were observed at ≥5 mg/kg bw per day in mouse dams exposed to PFOS on GD 1-17 (Thibodeaux et al., 2003). Total cholesterol and triglycerides were also decreased in mice exposed to 0.005% PFOS in feed (sole treatment group; approximately 6.5 mg/kg bw per day, using Health Canada's default assumption that 1 ppm in feed is equivalent to 0.13 mg/kg bw per day in mice [Health Canada, 1994]) (Qazi et al., 2010a).
In rats, the lowest LOAEL of 0.4 mg/kg bw per day was observed in dams exposed beginning 42 days prior to mating, until either gestational day 20 (for rats delivering by Caesarean section) or lactation day 4 (for rats delivering naturally; Luebker et al., 2005b). At this dose, total serum cholesterol was decreased; serum triglycerides were decreased only at ≥1.6 mg/kg bw per day. These effects were supported by decreased serum cholesterol (Seacat et al., 2003; Thibodeaux et al., 2003; Elcombe et al., 2012a) and decreased triglycerides (Thibodeaux et al., 2003; Elcombe et al., 2012a) in rats exposed to higher doses of PFOS.
Decreased liver cholesterol and triglycerides were also observed at the lowest dose in which this endpoint was studied (1.6 mg/kg bw per day, in rats dams in a developmental study; Luebker et al., 2005b).
9.2.2.4 Thyroid effects
A LOAEL of 0.15 mg/kg bw per day for altered thyroid hormone levels was observed in male and female Cynomolgus monkeys (4-6 animals per group) administered potassium PFOS (0, 0.03, 0.15, or 0.75 mg/kg bw per day) for 26 weeks by oral intubation of PFOS in a capsule (Seacat et al., 2002). Serum concentrations of TSH and free and total T3 and T4 were measured before treatment and at several time points during treatment (Day 37, 62, 91, and 182). The thyroid hormone changes considered consistent and statistically/biologically significant by the authors were increased TSH and decreased TT3 in males and females at 0.75 mg/kg bw per day. At 0.15 mg/kg bw per day, the changes observed included increased levels of TSH (males) and lower T3 concentrations (males and females). Some changes in T4 were also observed, but they were not consistent (including inconsistencies in direction). Based on the authors' statistical analysis, they stated that the LOAEL for thyroid hormone changes was 0.75 mg/kg bw per day (NOAEL = 0.15 mg/kg bw per day).However, as was described for serum lipid effects, Health Canada & EFSA performed reinterpretations of the Seacat et al. (2002) results. EFSA considered that the changes in thyroid hormones observed at 0.15 mg/kg bw per day were treatment-related and therefore concluded that it was justified to consider 0.03 mg/kg bw per day as a NOAEL (LOAEL of 0.15 mg/kg bw per day) (EFSA, 2008). Health Canada's reanalysis (2013c) was similar to that described for the serum lipid parameters (Section 9.2.2.3). Results from Dunnett's pair wise test comparing treatment groups to control group indicate a statistically significant difference at ≥ 0.15 mg/kg bw per day for decreased TT3 (both sexes) and decreased TT4 (females only). The time-dose interaction was significant for T3, T4 and TSH in males and for T4 in females (Health Canada, 2013c). Based on this statistical analysis, the LOAEL is considered to be 0.15 mg/kg bw per day for decreased TT3 and TT4 (NOAEL: 0.03 mg/kg bw per day).
The LOAEL for changes in thyroid hormones in rats was similar to that for monkeys. In a developmental study (Wang et al., 2011a), the effects were observed in rat dams exposed from GD 1 to PND 14 to 3.2 ppm of PFOS in feed (0.16 mg/kg bw per day using Health Canada's default assumption that 1 ppm in feed is equivalent to 0.05 mg/kg bw per day in rats [Health Canada, 1994], an assumption that might not be relevant in pregnant rats). The LOAEL was for dose-dependent decreases in T4; T3 was observed to be reduced only at a higher dose. In other rat studies of 5-91 days, decreases in T4 and T3 were observed, with the former effect being more sensitive (Yu et al., 2009a, 2011; Luebker et al., 2005b; Thibodeaux et al., 2003). The effects were primarily measured on total levels of the hormones, with some studies also measuring effects on levels of free T4 and T3. No effects on TSH were observed in rat studies.
Few mouse studies measured changes in thyroid hormones. A dose-dependent, but transient, decrease in total T4 was observed to be significant at 20 mg/kg bw per day in dams exposed GD 1-17 (Thibodeaux et al., 2003), but no effect on serum T3 or T4 levels was measured in mice exposed to up to 0.166 mg/kg bw per day for 28 days (Fair et al., 2011).
Changes in thyroid hormone levels were observed in rat and mouse pups exposed in utero to PFOS; these effects are described in Section 9.2.5.
No changes in thyroid follicular cell proliferation index was observed in male Sprague-Dawley rats exposed to K+PFOS (20 or 100 ppm in diet) for 7 days (1.9 or 9.6 mg/kg bw per day), when measured at various timepoints (1 day after cessation of exposure or after recovery periods of 28, 56, or 84 days (Elcombe et al., 2012a).
9.2.2.5 Other short-term effects
A wide variety of other short-term effects were observed for PFOS. These effects are described below, albeit only briefly as they occurred at higher levels than immune, hepatic, serum lipid, or thyroid effects.Decreased body weight (or body weight gain) was a common observation in a wide variety of studies. The effect was observed in rats at ≥0.4 mg/kg bw per day (Goldenthal et al., 1978a; Gortner, 1980; Wetzel, 1983; Christian et al., 1999; NOTOX, 1999; Grasty et al., 2003; Thibodeaux et al., 2003; Luebker et al., 2005a; Butenhoff et al., 2009; Cui et al., 2009; Kawamoto et al., 2011; Xia et al., 2011; Elcombe et al., 2012a); in mice at ≥0.4167 mg/kg bw per day (Yahia et al., 2008; Dong et al., 2009, 2011, 2012b; Era et al., 2009; Wan et al., 2011); and in rabbits at ≥1 mg/kg bw per day (Case et al., 2001). Contributing to this effect might be decreased food consumption, which was observed at ≥0.4 mg/kg bw per day in rats (Goldenthal et al., 1978a; Wetzel, 1983; Christian et al., 1999; Thibodeaux et al., 2003; Cui et al., 2009), ≥0.4167 mg/kg bw per day in mice (Yahia et al., 2008; Dong et al., 2011, 2012b), and ≥5 mg/kg bw per day in rabbits (Case et al., 2001).
Few studies demonstrated increases in mortality in adult animals. In monkeys, deaths were observed in all males in the 4.5 mg/kg bw per day group in a 90 day study (Goldenthal et al., 1978b), and in 2 (out of 6) males exposed to 0.75 mg/kg bw per day for 6 months (Seacat et al., 2002). Increased mortality was also observed in rats at ≥6 mg/kg bw per day (Goldenthal et al., 1978a; Wetzel, 1983; Grasty et al., 2005b; Cui et al., 2009), and in rabbits at ≥20 mg/kg bw per day (Case et al., 2001).
Other general effects observed included localized alopecia in rats (Christian et al., 1999) and soft stool, diarrhea, anorexia, emesis, and twitching, trembling and convulsions in monkeys (Goldenthal et al., 1978b).
A few neurotoxicity endpoints were observed in PFOS-exposed mice. Worsened performance was observed in neurobehavioural tests, including the water maze (at ≥2.15 mg/kg bw per day [Long et al., 2013], and 3 mg/kg bw per day, but not 6 mg/kg bw per day [Fuentes et al., 2007c]), and transient effects in the open field test (3 mg/kg bw per day) and number of rearings (6 mg/kg bw per day) (Fuentes et al., 2007c). However, the transient effects were both observed only on the same day, leading authors to conclude that the effects might be related to increased anxiety. Increased apoptosis (at ≥2.15 mg/kg bw per day) and glutamate levels (at 10.75 mg/kg bw per day) were observed in the hippocampus (Long et al., 2013). Increased expression of CaM-KIIα, pCREB, c-fos and c-jun was also observed in rat cortex and hippocampus at ≥1.7 mg/L in drinking water (0.238 mg/kg bw per day using Health Canada's default assumption that 1 ppm in water is equivalent to 0.14 mg/kg bw per day in rats [Health Canada, 1994]) (Liu et al., 2010a).
Neurotoxicity was also observed in rats. Reductions in activity and lethargy were observed at ≥5 mg/kg bw per day (Cui et al., 2009). Histological effects in the brain were also observed at ≥20 mg/kg bw per day (Cui et al., 2009), but not at levels of up to approximately 7 mg/kg bw per day (Kawamoto et al., 2011). Co-exposure with ultrasonic stimulation induced tonic convulsion (no tonic convulsion was induced by PFOS alone) after exposure to approximately 7 mg/kg bw per day (Kawamoto et al., 2011).
Renal effects from PFOS exposure were limited to increased BUN in male and female rats (with a LOAEL of 1.33 mg/kg bw per day; Seacat et al., 2003) and increased relative kidney weight at (at ≥5 mg/kg bw per day) in rats (Goldenthal et al., 1978a; Cui et al., 2009).
Respiratory tract effects-including pulmonary congestion, thickened epithelial walls, cell infiltration, and vasodilation-were observed at 5 and 20 mg/kg bw per day, with worsened severity at the high dose (Cui et al., 2009). Laboured breathing and bloodstains around the nose were also reported for the high dose in the study.
9.2.3 Long-term exposure and carcinogenicity
Only one chronic bioassay has been performed for PFOS. The study exposed Sprague-Dawley rats to dietary K+PFOS (0, 0.5, 2, 5 and 20 ppm in feed) for 2 years (mean daily doses: 0, 0.024, 0.098, 0.242 and 0.984 mg/kg bw per day for males; 0, 0.029, 0.120, 0.299 and 1.251 mg/kg bw per day for females) (Butenhoff et al., 2012b). A recovery group (20 ppm Rec.) was also exposed to the high dose diet for the first 52 weeks and then fed with control diet (mean daily doses: 1.144 and 1.385 mg/kg bw per day for male and female respectively). Early sacrifice was also performed at weeks 4 and 14; the observations at these time points are presented throughout Section 9.2 (as Seacat et al., 2003).Liver was identified as the principal target site in males (LOAEL of 0.5 ppm or 0.024 mg/kg bw per day; no NOAEL identified). The LOAEL was based on significant increased incidence of cystic degeneration in the liver in all dose groups. Other effects in the liver of males at higher doses (≥ 0.098 mg/kg bw per day) include significant increased incidence and severity of centrilobular hepatocytic hypertrophy, eosinophilic hepatocytic granule, centrilobular hepatocytic pigment, hepatocyte necrosis, and midzonal/centrilobular hepatocytic vacuolation. Most effects were observed to be reversible, as they were observed at levels similar to controls in the recovery group; however, incidence of cystic degeneration and hepatocyte necrosis were similar in the recovery group, indicating that the effect persisted even after exposures had been ceased for one year. Decreased mortality (statistically significant at 5 and 20 ppm) and changes in absolute and relative organ weights in the high dose group (increased in liver and decreased in spleen and left thyroid/parathyroid) were also observed. An increased incidence of interstitial fat infiltration was reported in males at 0.098 mg/kg bw per day (no data available for other doses).
In female rats, no consistent dose-response relationship was observed for non-neoplastic lesions in the liver; a statistically significant increased incidence of several liver lesions was observed at ≥0.120 mg/kg bw per day for lymphohistiocytic infiltrate, centrilobular hepatocytic hypertrophy, eosinophilic hepatocytic granule, centrilobular hepatocytic pigment, hepatocyte necrosis, periportal hepatocyte vacuolation, and macrophage pigmented infiltrate and decreased periportal hepatocyte hypertrophy. Increased relative (to body weight) brain, kidney, liver and spleen weight were also observed as well as decreased absolute left adrenal weight and relative (to brain weight) left and right adrenal weight.
Decreased serum total cholesterol was observed at several different timepoints in males (14, 17, and 53 weeks), with significance observed only at the high dose (NOAEL of 0.242 mg/kg bw per day, and LOAEL of 0.984 mg/kg bw per day). The effect in females was limited to a transient decrease, with decreases in the three highest dose groups at week 27 only.
Macroscopic observations of livers at the end of the study exhibited enlarged, mottled, diffusely darkened or focally lightened livers in male and female rats given 5 or 20 ppm. No data were available for the other groups (Butenhoff et al., 2012b).
Carcinogenic effects in the study included tumours in the liver, thyroid, and mammary gland. An increased incidence of total hepatocellular adenoma, statistically significant at 20 ppm, was observed in both sexes in rats exposed for 2 years, but not 52 weeks. Thyroid follicular cell tumours (adenomas in males, and adenomas/carcinomas combined in females) were significantly increased in recovery group males and in the second highest exposure group in females (5 ppm or 0.299 mg/kg bw per day). In females, mammary fibroadenoma and fibroadenoma/adenoma combined were increased over controls only in the lowest dose group, and showed a significant negative trend.
The authors noted that serum and liver PFOS levels at the end of study were dramatically lower than after 14 weeks exposure in both sexes. In males, serum levels at terminal sacrifice were 33%, 44%, 51%, and 47% of those measured on Week 14 in the 0.5, 2, 5, and 20 ppm groups, and liver levels were 33%, 36%, 19%, and 33% of Week 14 values in the same dose groups, respectively. The authors suggested that this decline was likely due to chronic progressive nephritis leading to increased urinary excretion of PFOS across all treatment groups. Data on nephritis were not provided by authors, who described the effect as occurring across all treatment groups; however, they stated significant associations were observed between incidence and severity of nephritis in males (but only at one dose in females). Serum PFOS concentrations also increased in approximate proportion to length of dosing between Weeks 4 and 14; however, Week 53 concentrations in the 20 ppm group were similar to those measured on Week 14, suggesting that steady state may have been approached after 14 weeks in the 20 ppm dose group. The serum PFOS levels corresponding to the LOAEL (0.5 ppm in diet) were 4,040 ng/mL at 14 weeks and 1,310 ng/mL at 105 weeks (Butenhoff et al., 2012b).
9.2.4 Genotoxicity
Based on negative results of a large series of in vitro and in vivo short-term tests of genes, chromosomes, or DNA repair, EFSA (2008) and Health Canada (2006) concluded that PFOS and its salts are not genotoxic. More recently published data (see the following subsections) are in agreement with this conclusion.9.2.4.1 In vitro findings
Negative results were obtained in various in vitro assays conducted for PFOS on prokaryotes, namely the reverse gene mutation assay in Salmonella typhimurium (TA100, TA1535, TA1537, TA1538 and TA09 strains; 2 studies) and Escherichia coli (WP2uvrA, one study) conducted with/without metabolic activation (S9) and the mitotic recombination test in Saccharomyces cerevisiae (D4 strain, 1 study) (as reviewed by EFSA, 2008). PFOS (tested up to 1,000 µM) had no mutagenic activity in the umu test (Oda et al., 2007).In human hepatoma HepG2 cells, PFOS (up to 400 µM for 24 h) did not induce ROS generation, DNA single strand breaks or micronuclei (Florentin et al., 2011). In another study of human HepG2 cells, PFOS induced slight ROS generation (0.4-2,000 µM) without generating detectable DNA damage (200 µM) (Eriksen et al., 2010).
PFOS did not induce chromosomal aberrations in cultured human lymphocytes with or without metabolic activation and did not induce unscheduled DNA synthesis (UDS) in primary cultured rat liver cells (as reviewed by EFSA, 2008).
In Syrian hamster embryo (SHE) cells, PFOS induced cell transformation at non-cytotoxic concentrations (0.2-2 µg/mL) and increased the expression of PPARβ/δ (0.2 µg/mL for 1 and 7 days; 2 µg/mL for 7 days), PPARγ (0.02-2 µg/mL for 7 days) and PPARα (20 µg/mL for 7 days). PFOS did not induce DNA damage in the comet assay (Jacquet et al., 2012).
Negative results were also found in different in vitro tests conducted with several PFOS precursors (as reviewed by EFSA, 2008).
9.2.4.2 In vivo findings
PFOS was negative in the in vivo bone marrow mouse micronucleus assay at single oral doses of 237.5, 450 and 950 mg/kg bw (with sampling at 24, 48 and 72 hours), and several PFOS precursors were found negative in different in vivo tests (as reviewed by EFSA, 2008).A comet assay conducted in Paramecium caudatum was negative (Kawamoto et al., 2010).
9.2.5 Reproductive and developmental toxicity
The reproductive and developmental database for PFOS is robust. A 2-generational study has been developed in rats (Christian et al., 1999; Luebker et al., 2005a), and reproductive and developmental parameters have been investigated in many one-generation studies in rats, mice, and rabbits. Effects that occurred at the lowest levels in animals exposed in utero included changes in brain structure (≥0.1 mg/kg bw per day), neurobehaviour (≥0.3 mg/kg bw per day), thyroid hormone levels (≥0.16 mg/kg bw per day), and fetal body weight (≥0.1 mg/kg bw per day). The majority of the other effects were observed at ≥1 mg/kg bw per day. Doses described throughout this section refer to maternal doses for animals exposed in utero, unless otherwise specified.Changes in structure and in levels of various proteins and neurotransmitters in the brain were observed in mice and rats. In Sprague-Dawley rats exposed on GD0-20 to 0, 0.1, 0.6, and 2 mg/kg bw per day by gavage, structural modification of synapses in the hippocampus was observed in all doses (Zeng et al., 2011b). This study also identified decreased mRNA levels of synapsin1, synapsin2, and synaptophysin in the brain at all doses. Brain transcriptional changes-with multiple genes related to long-term potentiation/depression, synaptic transmission, calcium-dependent signal transduction, and phosphatidylinositol signalling pathways-were also observed in rats exposed to 3.2 ppm of PFOS in feed (equivalent to approximately 0.16 mg/kg bw per day using the Health Canada default assumption of 1 ppm in food = 0.05 mg/kg bw per day in rats [Health Canada, 1994]) (Wang et al., 2010, 2012). A companion study exposing rats to the same doses on GD2-21 also noted increases in GFAP in the hippocampus and cortex (at ≥0.1 mg/kg bw per day) and IL-1β and TNF-α in the hippocampus (at ≥0.6 mg/kg bw per day)(Zeng et al., 2011a). Changes were observed in the levels of proteins that are important in brain development (CamKII, GAP-43, synaptophysin, and tau, in cerebral cortex and/or hippocampus), in mice exposed to a single dose of 8.7 mg/kg (Johansson et al., 2009). At higher doses (>1 mg/kg bw per day) in rats, changes were observed in choline acetyltransferase activity in prefrontal cortex (Lau et al., 2003) and calcium related signalling molecules (Liu et al., 2010b).
In addition to changes in the brain, adverse effects from PFOS manifested as neurobehavioural changes at ≥0.3 mg/kg bw per day. The most common effect observed was changes in activity levels in mice (Onishchenko et al., 2011; Johansson et al., 2008; Fuentes et al., 2007a) and rats (Butenhoff et al., 2009), with some evidence that the effect was more pronounced in males (Onishchenko et al., 2011). Other effects observed were neuromotor decrements (worsened screen climb and pull and diminished forelimb grip strength in mice [Fuentes et al., 2007b] and decreased hindlimb strength in rats [Butenhoff et al., 2009]), delayed reflex in rats (Christian et al., 1999; Luebker et al., 2005a), and decrements in spatial learning and memory in the water maze test (Liu et al., 2009b) and hidden platform test (Wang et al., 2015) in rats.
Developmental exposure to PFOS resulted in changes to thyroid hormones at similar exposure levels to adult animals. Rat neonates whose mothers were exposed during gestation and lactation to ≥3.2 ppm in food (equivalent to ≥0.16 mg/kg bw per day using Health Canada's default assumption that 1 ppm in food = 0.05 mg/kg bw per day [Health Canada, 1994], an assumption that might not be relevant in pregnant or newborn rats) had dose-dependent decreases in total T4 (Wang et al., 2011a; Yu et al., 2009b; Lau et al., 2003). No changes in T3 levels were observed in two rat studies (Yu et al., 2009; Lau et al., 2003), but they occurred at high levels in another (Wang et al., 2011a). An additional neonatal study describing thyroid effects did not see any change in serum TSH in rat offspring, but increases in thyroid follicular epithelial cell proliferation were observed at 1 mg/kg bw per day (Chang et al., 2009). The only effect observed in developmental studies in mice was a decrease in total T4 at ≥5 mg/kg bw per day, but the effect was less consistent than in rats (Lau et al., 2003).
Although effects on the immune system and serum lipid levels were key effects that were well studied in adult mice, rats, and monkeys, very few studies investigated these effects during prenatal exposure. Adult mice exposed in utero to PFOS at 0, 0.1, 1, or 5 mg/kg bw per day had decreases in NK cell activity (at ≥1 mg/kg bw per day in males and 5 mg/kg bw per day in females), decreases in SRBC-IgM response (in males at 5 mg/kg bw per day), and changes in thymic lymphocytic subpopulations (at 5 mg/kg bw per day)(Keil et al., 2008). Increased serum cholesterol and LDL were increased on GD21 in rat fetuses exposed in utero to ≥1.6 mg/kg bw per day, the lowest dose at which this effect was studied (Luebker et al., 2005b). At LD5, the same study found no effects on serum lipid parameters in pups, but did observe a decrease in liver triglyceride levels.
Increased absolute or relative fetal liver weight was increased in mice at ≥5 mg/kg bw per day (Keil et al., 2008; Lau et al., 2003; Abbott et al., 2009). The effect was also observed in rats (Lau et al., 2003), but the species was less sensitive than mice (effects were only observed at 20 mg/kg bw per day.
Cardiac mitochondrial injury and increased relative heart weight were observed at 2 mg/kg bw per day in rats exposed in utero (Xia et al., 2011). These effects were not accompanied by changes in heart rate or blood pressure; however, systolic blood pressure was increased in rats exposed prenatally to 18.75 mg/kg bw per day (Rogers et al., 2014). The blood pressure effect in Rogers et al. (2014) was correlated with decreased nephron endowment. The only other effect that was noted in kidney development was decreased kidney weight in mice exposed to 5 mg/kg bw per day (Keil et al., 2008).
Changes suggesting immaturity of lungs were noted in rats exposed in utero to PFOS at ≥2 mg/kg bw per day. Observed histological changes included hemorrhage, thickened interalveolar septum and alveolar walls, focal lung consolidation, inflammatory cell infiltration, and apoptotic cells (Chen et al., 2012; Grasty et al., 2003, 2005). Atelectasis and abnormal expansion of lungs was also observed (Grasty et al., 2003, 2005). Because the pulmonary surfactant profile in offspring was normal and rescue agents (accelerators of pulmonary maturation) did not improve laboured respiration and mortality, the authors thought the latter effects were not due to lung immaturity (Grasty et al., 2005). The only evidence of potential adverse lung effects in mice pups was the observation of post-delivery cyanosis in some pups exposed to 12.5 mg/kg bw per day (Borg et al., 2010).
A variety of adverse effects were noted on the general health status of animals exposed prenatally to PFOS. Decreased survival or viability, or increased mortality were observed in foetuses or pups at ≥1.6 mg/kg bw per day in rats (Christian et al., 1999; Lau et al., 2003; Luebker et al., 2005a, 2005b; Xia et al., 2011) and at ≥4.5 mg/kg bw per day in mice (Lau et al., 2003; Yahia et al., 2008; Abbott et al., 2009). Decreased pup body weight or fetal weight was observed at the lowest levels at ≥0.1 mg/kg bw per day in a study of rats (Christian et al., 1999; Luebker et al., 2005a, 2005b). Other studies noted effects at higher levels in rats (at ≥1.6 mg/kg bw per day; Wetzel, 1983; Lau et al., 2003; Wang et al., 2011a; Xia et al., 2011; Chen et al., 2012; Rogers et al., 2014), mice (at 6 mg/kg bw per day; Fuentes et al., 2007b; Era et al., 2009), and rabbits (at ≥2.5 mg/kg bw per day; Case et al., 2001). Non-significant growth deficit was also observed in mice at 10 mg/kg bw per day (Lau et al., 2003). Rat pups also appeared pale and delicate at 1.6 mg/kg bw per day (Wang et al., 2011a).
Prenatal PFOS exposure caused developmental landmark delays and structural abnormalities. Delayed eye opening was observed at ≥1 mg/kg bw per day in mice (Lau et al., 2003; Fuentes et al., 2007b; Abbott et al., 2009) and ≥1.6 mg/kg bw per day in rats (Christian et al., 1999; Lau et al., 2003; Luebker et al., 2005a); moreover, abnormalities were observed in the eye lens of rats at ≥1 mg/kg bw per day (Gortner, 1980). Other developmental landmark delays occurred in pinna unfolding in rats (Christian et al., 1999; Luebker et al., 2005a) and mice (Fuentes et al., 2007b), and incisor eruption in mice (Fuentes et al., 2007b; Yahia et al., 2008;). Skeletal abnormalities were also observed at ≥1 mg/kg bw per day. The effects observed at the lowest levels were sternal defects in mice (Lau et al., 2003; Yahia et al., 2008;) and rats (Wetzel, 1983; Lau et al., 2003;) and incomplete skull closure in rats (Wetzel, 1983). Other effects observed included: cleft palate in mice (Lau et al., 2003; Yahia et al., 2008; Era et al., 2009) and rats (Wetzel, 1983; Lau et al., 2003); rib abnormalities in mice (Yahia et al., 2008) and rats (Wetzel, 1983); delayed ossification in mice (Yahia et al., 2008), rats (Wetzel, 1983), and rabbits (Case et al., 2001); curved fetus, spina bifida occulta, and tail abnormalities in mice (Yahia et al., 2008); and subcutaneous edema and cryptorchism in rats (Wetzel, 1983).
Decreases in reproductive organ weights were observed in animals. A dose-related trend in decreased uterus weight was observed in mice, and was significant at ≥0.166 mg/kg bw per day (Fair et al., 2011). Reduced mean gravid uterine weight was also observed at 10 mg/kg bw per day in rats (Wetzel, 1983). These effects occurred in the absence of histological effects. Other reproductive organ weight decreases that were observed were in male rats, affecting absolute seminal vesicle weight (Christian et al., 1999) and relative gonad weight (Cui et al., 2009).
Reproductive effects also resulted from PFOS exposure. The effect at the lowest level was decreased gestation length at ≥0.8 mg/kg bw per day in rats (Christian et al., 1999; Luebker et al., 2005a, 2005b). Other effects observed at higher levels were fewer implantation sites in rats (Luebker et al., 2005a; Christian et al., 1999), reduced litter size in rats (Christian et al., 1999; Xia et al., 2011) and rabbits (Case et al., 2001), and increase in fetal resorptions, dead foetuses, and stillbirths in rats (Luebker et al., 2005a; Wetzel, 1983), rabbits (Case et al., 2001), and mice (Lau et al., 2003; Yahia et al., 2008). A significantly reduced lactation index was also observed in rat pups exposed to 1.6 mg/kg bw per day (Christian et al., 1999; Luebker et al., 2005a).
Reproductive effects observed in males were limited to decreased estradiol in male monkeys at 0.75 mg/kg bw per day (Seacat et al., 2002), and decreased serum testosterone levels and epididymal sperm count in male rats exposed to 10 mg/kg bw per day (Wan et al., 2011).
Comparing observations across generations in two-generation studies can help to identify emerging patterns in developmental and reproductive effects. Effects in rat pups from the two-generation study (Christian et al., 1999; Luebker et al., 2005a) that were described throughout this section occurred primarily in the F1 offspring. The F0 dams were exposed to four different doses of PFOS-0.1, 0.4, 1.6, or 3.2 mg/kg bw per day-for 42 days prior to mating, through the mating period (maximum of 14 days), and to day 9 of gestation for rats with C-section delivery, or lactation day 20 for rats with natural delivery. Significantly decreased viability of F1 pups was observed at the two higher doses. At 3.2 mg/kg bw per day, fewer pups were liveborn and more were stillborn. Moreover, the numbers of pups that survived in the first few days of life was lower in the 1.6 mg/kg bw per day group. At the high dose, 0% of pups survived, and at 1.6 mg/kg bw per day, survival was only 66.1% (compared with >98% in controls and lower dose groups). Delays in developmental landmarks-eye opening, pinna unfolding, surface righting, and air righting-were also observed at 1.6 mg/kg bw per day in F1 pups. Maternal toxicity had been apparent in these dose groups, as F0 dams displayed decreases in food consumption and bodyweight gain at ≥0.4 mg/kg bw per day. Due to significant decreases in survival at the two highest doses, exposure to F1 dams was limited to 0.1 and 0.4 mg/kg bw per day. No effects on reproductive outcome (including number of live births and pup survival) were observed in F1 dams/F2 pups; moreover, no maternal toxicity was observed. The only effect observed in the F2 generation was a decrease in pup weight and weight change at 0.4 mg/kg bw per day, for 2 timepoints only (days 7 and 14). Therefore, decreased pup weight was first observed at a lower level in F2 pups (0.4 mg/kg bw per day) than F1 pups (1.6 mg/kg bw per day); however, the effect was less severe and/or more transient than in F1 pups, where the effect was observed consistently at all time points (days 1-21). Mating and fertility appeared to be unaffected in both generations.
9.3 Mode of action
Mode of action (MOA) analysis was considered for effects occurring at the lowest PFOS levels (i.e. immune effects in mice, lipid effects in monkeys and mice, liver weight increase in rats and mice, liver histological changes in rats, hepatocellular tumours in rats, and thyroid hormone changes in monkeys, rats, and mice). Only a preliminary evaluation of data could be performed for most of the MOAs; a MOA analysis using recent guidance (Meek et al., 2014) could only be performed for peroxisome proliferation effects on liver endpoints. Based on the MOA analysis, no endpoints were considered to be irrelevant to humans, and the results suggest that the TDI approach is the most appropriate method for cancer risk assessment. Results of the MOA evaluations are summarized in this section.9.3.1 Direct-acting mutagenicity
Direct-acting mutagenicity was considered as a potential MOA for the development of hepatocellular tumours in rats. As discussed in Section 9.2.4, evidence strongly indicates that PFOS is not mutagenic, with or without metabolic activation. PFOS was negative for genotoxicity in a wide variety of in vitro and in vivo assays. The pattern of PFOS-induced tumours also did not follow that of typical mutagens. For example, mutagens are typically expected to cause tumours in many different organs, but PFOS only induced tumours in the thyroid and liver of rats (Butenhoff et al., 2012b), which were organs that also demonstrated adverse non-cancer effects in rats and other species. Furthermore, mutagens often produce a high incidence of tumours, which occur at early timepoints. In PFOS-exposed animals, hepatocellular tumours were only observed after lifetime duration (and not in rats exposed for 1 year and kept alive for an additional 1 year), and at low incidences (8-12%) (Butenhoff et al., 2012b). Thyroid tumours in the study demonstrated inconsistencies in time-dose relationships and dose-response relationships (with a significant increase only in the high-dose group exposed for 1 year and not 2 years in males, and a significant increase in a middle dose group in females). The incidence of tumours was still relatively low in most groups, ranging from 7-10% when observed in rats exposed for two years (6-10%), and slightly higher in the male recovery group (23%). These data suggest that low-dose linear extrapolation is not appropriate for PFOS-induced tumours. No further MOA analysis is required for direct-acting mutagenicity, unless contradictory data are published.9.3.2 Peroxisome proliferation
Peroxisome proliferation was considered as a potential MOA for hepatocellular tumours in rats, hepatic cystic degeneration in rats, liver weight increase in mice, and increase in serum lipids in monkeys and mice. Some data exist for hepatic PPAR activity in rats; however, no studies directly measured PPAR impact on other outcomes. As insufficient data were available to fully apply the evolved Bradford-Hill considerations to evaluate the MOA, the weight of evidence analysis is limited to the evaluation of the dose-response of key events for peroxisome proliferation in rat liver.Three main key events in the peroxisome proliferation MOA are considered to lead to liver histological effects and hepatocellular tumours. These key events are 1) the activation of hepatic PPARα receptors, which leads to 2) altered cell growth pathways that inhibit apoptosis and/or promote cell replication, eventually leading to 3) hepatocyte proliferation (Corton et al., 2014).
9.3.2.1 Key event 1 - PPARα activation
The lowest doses at which PPARα activation was investigated were 0.024-1.25 mg/kg-bw per day, in rats exposed for 4, 14, or 104 weeks (Seacat et al., 2003; Butenhoff et al., 2012b). PPARα activation was not observed to occur at any dose, based on the absence of increase in palmitoyl CoA oxidase activity. Increased PPARα activity was measured after 1 week of exposure at higher doses in rats, with a dose-dependent upregulation of PPARα target genes after exposure to 5 or 20 mg/kg-bw per day (Ye et al., 2012), and an increase in ACOX and 12-OH LAH at 9.65 mg/kg-bw per day (Elcombe et al., 2012b). However, the latter effects were not observed after 1 week of exposure to 1.93 mg/kg-bw per day (Elcombe et al., 2012b). PPAR‑activation only occurs at higher concentrations for PFOS than PFOA in in vitro (Takacs and Abbott, 2007; Rosen et al., 2013) and in vivo (Rosen et al., 2010) gene expression studies. Furthermore, studies with PPAR-null mice indicated histological effects and hepatic gene expression changes that were similar to mice with active PPAR activity, indicating PPAR-independent effects (Rosen et al., 2010).9.3.2.2 Key event 2 - altered cell growth
No studies of markers of altered cell growth pathways could be found.9.3.2.3 Key event 3 - hepatocyte proliferation
Hepatocyte proliferation was not observed at the lowest doses studied (0.024-1.25 mg/kg bw per day, for 4, 14, or 104 weeks; Seacat et al., 2003; Butenhoff et al., 2012b). The liver proliferative index was increased, but was not sustained, after exposure to 1.93 or 9.65 mg/kg bw per day for 1 week (Elcombe et al., 2012b).9.3.2.4 Comparison of dose-response of key events and outcomes
For modes of action to be deemed as relevant for an adverse outcome, dose-response concordance-i.e., the observation of early key events at doses that are lower than or equal to later key events and the adverse outcome-is required. For PFOS, however, the lowest observed doses associated with the adverse outcomes in rats (cystic degeneration: 0.024 mg/kg bw per day, hepatocellular adenomas: 0.984 and 1.251 mg/kg bw per day in male and females, respectively) were lower than those at which hepatocyte proliferation was observed (≥1.93 mg/kg bw per day). This hepatocyte proliferation also might not be associated with PPARα activation, which was only observed at ≥5 mg/kg bw per day. Finally, PPARα activation and hepatocyte proliferation were not observed concurrently with cystic degeneration and hepatocellular adenoma in the study where these effects were observed. Because it appears that liver proliferation, hepatocellular adenomas, and cystic degeneration precede PPARα activation, adverse hepatic effects observed in rats exposed for 2 years to PFOS do not appear to be driven by a peroxisome proliferation MOA. For this reason, human relevance of PFOS-induced hepatic effects cannot be discarded. Moreover, hepatic effects do not appear to be specific to rodents-the LOAEL for hepatocellular hypertrophy accompanied by cytoplasmic vacuolation in monkeys (0.75 mg/kg bw per day; Seacat et al., 2002) is on the same order of magnitude as in rats (0.242 mg/kg bw per day; Butenhoff et al., 2012b).Although insufficient data exist to examine the impact of PPAR activation on changes in serum lipid, thyroid, and immune parameters, peroxisome proliferation is plausible for all endpoints. PPAR activators are known to produce hypolipidemic effects (Corton et al., 2014), and PFOS is similar in structure to fatty acids. In rats, PFOS-induced alterations in gene expression related to fatty acid metabolism and thyroid hormone synthesis and release were of a similar pattern to known PPARα activators (Martin et al., 2007). Some immune effects (weight and cellularity changes in spleen and thymus) were muted in PPAR-null mice (vs. wild-type mice) exposed to PFOA; however, no similar data exist for PFOS, which does not appear to be as strong of a PPAR activator as PFOA. The peroxisome proliferation MOA for these endpoints cannot be fully examined until further data are produced on the impacts of PFOS-induced PPAR activation on immune and serum lipid pathways.
9.3.3 Sex hormone disruption
Sex differences were observed in immune response, with males more sensitive than females. However, no studies have been developed to identify whether this effect is associated with sex hormones; therefore, there are insufficient data to evaluate the MOA. PFOS does, however, appear to have some impact on sex hormone disruption-in a variety of in vitro assays of estrogenicity, PFOS did not display direct estrogenic activity, but enhanced the effects of 17β‑estradiol in several assays (Sonthithai et al., 2015). If more in-depth studies on the effect of PFOS on sex hormone disruption are performed, this potential MOA could be further investigated.9.3.4 Immune suppression
Immune suppression in rats (decrease in IgM and NK cell levels) has been observed at lower doses than those that were tumorigenic. Although NK cells are involved in eliminating cancer cells, no studies investigating the role of PFOS-induced immunosuppression in tumour development have been performed. No detailed analysis for this potential MOA can be performed using current data. If more in-depth studies on the association between PFOS-induced immunosuppression and tumour development are performed, this potential MOA could be further investigated.9.3.5 Other MOAs
Insufficient data exist to allow for the assessment of other potential MOAs considered in the MOA analysis. Some data-particularly in regards to PPAR activation/peroxisome proliferation-exist for other endpoints that were not included in the MOA analysis (i.e. effects that were observed only at higher PFOS exposure levels).10.0 Classification and assessment
The benchmark dose (BMD) approach was used wherever possible to calculate potential points-of-departure, because it is derived on the basis of data from the entire dose-response curve for the critical effect rather than from the single dose group at the NOAEL (IPCS, 1994). A lower confidence limit of the benchmark dose (BMDL) has been suggested as an appropriate replacement of the NOAEL (Crump, 1984). More specifically, a suitable BMDL is defined as a lower 95% confidence limit estimate of dose corresponding to a 1-10% level of risk over background levels. Definition of the BMD as a lower confidence limit accounts for the statistical power and quality of the data (IPCS, 1994). For dichotomous data (i.e. presence vs. absence of effect), benchmark dose values representing a 10% increase in adverse effect over background rates (BMD10) and their lower 95% confidence limits (BMDL10) were calculated using the U.S. EPA Benchmark Dose Software (BMDS Version 2.6.0.86) (U.S. EPA, 2015).Large pharmacokinetic differences exist between animals and humans, with lower clearance (i.e., higher half-life values) in humans than in rats, mice, and monkeys. These differences result in higher target tissue doses in humans when exposed to the same external doses as animals. Default approaches for interspecies extrapolation (e.g., using an interspecies uncertainty factor of 10 or allometric scaling) are therefore not considered to be sufficiently protective of humans. As described in Section 8.6, AKUF values (i.e., the component of the CSAF reflecting interspecies toxicokinetic differences) were calculated with a PBPK model to address pharmacokinetic differences between animals and humans. As different AKUF values were calculated for various doses, the values can also address non-linearities in pharmacokinetics. Weaknesses still exist with this approach, as outlined in detail in Section 8.6, including the use of the steady-state plasma concentration as a dose metric, rather than selecting organ-based values or exploring whether other dose metrics (e.g., peak concentrations) are more appropriate. However, despite the weaknesses, the approach was determined to be the best of the available options. This approach was selected over the use of serum concentrations as PODs, because human PBPK models were determined to not be sufficiently robust for precise estimates of human exposure levels corresponding to serum-based PODs.
10.1 Cancer risk assessment
The carcinogenicity of PFOS has not been evaluated by IARC. Consistent observations of associations between PFOS exposure and cancers have not been identified in epidemiological studies. Some associations between PFOS and risk of cancer of the bladder, breast, male reproductive organs, and overall cancers were observed; however, the evidence does not support the carcinogenicity of PFOS. The association for bladder cancer was lost after further follow-up, the population demonstrating associations for breast cancer were also exposed to several other chemicals, and associations for male reproductive cancers were not supported by studies of other populations. Therefore, although some evidence of an association between PFOS and the risk of cancer has been observed, the effects were equivocal, and no clear trend could be determined due to limitations in the studies (including small number of cases, confounding, and participant selection bias).In the sole chronic bioassay performed for PFOS, tumours were observed in the liver, thyroid, and mammary gland of Sprague-Dawley rats (Butenhoff et al., 2012b). Hepatocellular adenoma was observed to be significantly increased in high dose males and females (0.984 and 1.251 mg/kg bw per day, respectively) after 2 years of exposure. Dose-response and temporal patterns for thyroid and mammary gland tumours were less consistent. Thyroid follicular cell adenomas were increased in recovery group males (exposed to 1.144 mg/kg bw per day for 52 weeks), but not those exposed for the duration of the study. Combined thyroid follicular cell adenomas and carcinomas were increased only in females in the second-highest exposure group (0.299 mg/kg bw per day), but not in high-dose females. Finally, incidence of mammary fibroadenoma and combined fibroadenoma/adenoma was increased only in the lowest dose of females (0.029 mg/kg bw per day), but no other groups.
Although the mode of action for PFOS-induced tumours has not yet been elucidated, the weight of evidence more strongly suggests that PFOS is a non-mutagenic compound (see Sections 9.2.4 and 9.3). For this reason, the tolerable daily intake (TDI) approach is the most appropriate method for deriving a health-based value (HBV) for cancer.
Hepatocellular tumours were selected as the critical effect for the cancer risk assessment, as it is the cancer endpoint with the most consistent dose-response relationship. In males, tumours were only classified as hepatocellular adenomas; in females, the majority of tumours were hepatocellular adenomas, with one incidence of hepatocellular carcinoma in the 2-year high-dose group. Incidence of these tumours in male and female rats is presented in Table 3.
| Treatment group (ppm) | Males - hepatocellular adenomas only | Females - hepatocellular adenoma & carcinoma combined | ||
|---|---|---|---|---|
| Dose (mg/kg bw per day) | Incidence (%) | Dose (mg/kg bw per day) | Incidence (%) | |
| 0 | 0 | 0/60 (0%) | 0 | 0/60 (0%) |
| 0.5 | 0.024 | 3/50 (6%) | 0.029 | 1/50 (2%) |
| 2 | 0.098 | 3/50 (6%) | 0.12 | 1/49 (2%) |
| 5 | 0.242 | 1/50 (2%) | 0.299 | 1/50 (2%) |
| 20 | 0.984 | 7/60 (12%)Footnote * | 1.251 | 5/60 (8%)Footnote * |
| 20 ppm recovery | 1.144 | 0/40 (0%) | 1.385 | 2/40 (5%) |
To reflect the large interspecies differences in pharmacokinetics, the human-equivalent point-of-departure (PODHEQ) can be calculated by dividing the BMDL10 by the AKUF, as follows:
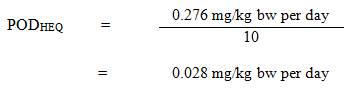
Figure 3 - Text Equivalent
- 0.276 mg/kg bw per day is the BMDL10 for hepatocellular tumours in male rats (Butenhoff et al., 2012b); and
- 10 is the dose-specific AKUF for rats in the 0.1 mg/kg bw per day range (see Section 8.6.2).
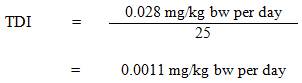
Figure 4 - Text Equivalent
- 0.028 mg/kg bw per day is the PODHEQ calculated above; and
- 25 is the composite uncertainty factor, as described below.
Using this TDI, the HBV for drinking water can be calculated as follows:
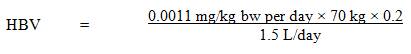
Figure 5 - Text Equivalent
- 0.0011 mg/kg bw per day is the TDI derived above;
- 70 kg is the average body weight of an adult;
- 0.2 is the default allocation factor for drinking water, used as a "floor value", since drinking water is not a major source of exposure and there is evidence of widespread presence in at least one of the other media (air, food, soil, or consumer products) (Krishnan and Carrier, 2013); and
- 1.5 L/day is the daily volume of water consumed by an adult; dermal and inhalation exposures from bathing and showering are not considered to be significant routes of exposure (as described in Section 5.7).
10.2 Non-cancer risk assessment
Although epidemiological evidence has shown an association between the exposure to PFOS and an increased risk of multiple health outcomes, such as reproductive, developmental, and immunological effects (see Section 9.1.3), a point-of-departure (POD) cannot be derived from the studies due to their limitations, including in terms of study design, bias and confounders.The effect observed at the lowest exposure levels was immune system suppression in mice. The lowest LOAEL for immunosuppression data classified by IPCS (2012) as providing the strongest weight of evidence for immunotoxicity was suppression of SRBC-specific IgM in mice at ≥0.00166 mg/kg bw per day (Peden-Adams et al., 2008). Immune system effects were excluded from the quantitative risk assessment due to inconsistencies in NOAELs and LOAELs among studies and uncertainty of the importance of observed effects to human health, both of which will be expanded upon in this section. Inconsistencies were observed in the effective PFOS doses for immune function endpoints: the suppression of SRBC-specific IgM in B6C3F1 mice in Peden-Adams et al. (2008) was observed at ≥0.00166 mg/kg bw per day, whereas the LOAEL in C57Bl/6 mice was 0.0833 mg/kg bw per day, with no significant changes observed at 0.0083 mg/kg bw per day (Dong et al., 2009) or 0.0167 mg/kg bw per day (Dong et al., 2011). Moreover, NK cell activity was actually increased at 0.0166 mg/kg bw per day in B6C3F1 mice (Peden-Adams et al., 2008); in C57Bl/6 mice, the effect was non-monotonic, with increased activity at low doses (significant at 0.0833 mg/kg bw per day), and significant decreases at higher doses (0.833 and 2.083 mg/kg bw per day) (Dong et al., 2009). An additional study identified increased mortality from influenza A infection in mice at 0.025 mg/kg bw per day (Guruge et al., 2009); however, this effect was not studied in other species. The adversity of IgM suppression and changes in NK cell activity is also debatable-although these effects indicate immune system changes, it is unclear whether small variations in these measures are sufficient to result in adverse health effects in humans. Of note for discussion of clinical importance in humans is the Grandjean et al. (2012) study, which demonstrated that despite decreased vaccine-specific immunoglobulin response in PFOS-exposed children, the number of children with immunoglobulin levels below the clinically-protective level was low. Moreover, mice appear to be more sensitive than other species, as the LOAEL for immunosuppression was several orders of magnitude higher in the lone rat study, at 3.21 mg/kg bw per day (Lefebvre et al., 2008). In humans, evidence of immunosuppression is inconsistent-associations are observed between PFOS levels and decreases in antibodies against some (but not all) illnesses, and the influence of PFOS exposure on clinical immunosuppression (i.e. incidence of illnesses) appears to be more tenuous. Therefore, although low PFOS doses appear to be associated with immunosuppression, the data are not considered to be presently reliable for use as a key study for the PFOS assessment. Further explorations should be performed to address the nearly two orders of magnitude difference in LOAELs in the studies before these endpoints can be reliably considered as a basis for a risk assessment.
The adverse effect observed at the lowest level (other than the IgM and NK-cell effects in Peden-Adams et al., 2008) was liver cystic degeneration observed in male Crl:CD(SD)IGS BR rats exposed for 104 weeks in feed (Butenhoff et al., 2012b). This effect was observed in a robust study, with 4 treatment groups (n ≥ 55) and an additional high-dose recovery group (n = 40); however, it is not proposed as critical effect for the risk assessment. The endpoint is a common benign, spontaneous lesion in aging rats, particularly males (Bannasch and Zerban, 1997; Karbe and Kerlin, 2002), which has been classified by some pathologists as a benign neoplasm (Bannasch, 2003). Cystic degeneration only rarely occurs in other mammals, including humans (Bannasch and Zerban, 1997; Karbe and Kerlin, 2002). Although cystic degeneration can be associated with foci of cellular alteration or tumours (Karbe and Kerlin, 2002), the progression in PFOS-exposed animals does not follow that of the more serious histological changes. Moreover, the incidence of cystic degeneration was similar among exposure groups, ranging from 27-38% with no dose-related increases, and occurring in similar levels in high-dose rats exposed for 2 years versus recovery rats (33 vs. 38%). The frequency of cystic degeneration in most groups is within the range of spontaneous development in male rats, which is as high as 34% (Karbe and Kerlin, 2002).
Hepatocellular hypertrophy was first observed at one dose higher than the LOAEL for cystic degeneration (0.098 mg/kg bw per day) in the Butenhoff study, and incidence increases in a dose-related manner. Although hepatocellular hypertrophy can sometimes be considered an effect that is adaptive rather than adverse in its own right, evidence of other histological effects in the liver at higher concentrations provide an indication of their progression upon continued exposure (ECETOC, 2002; Hall et al., 2012). Clearly adverse histological effects (including cytoplasmic vacuolation) were observed in livers of male rats beginning at the next dose level (0.242 mg/kg bw per day) in the study. Although hepatocellular hypertrophy occurs at one dose level lower than the clearly adverse histological effects, the effect is proposed as a critical effect for this assessment, as it might be a sensitive indicator of the potential for the progression of adverse histological effects. Moreover, the LOAELs were the same for both hepatocellular hypertrophy and cytoplasmic vacuolation in rats from the Butenhoff study that were sacrificed early (after 4 or 14 weeks, with LOAELs of 0.34-0.37 mg/kg bw per day; Seacat et al., 2003) and female rats exposed for 2 years (0.299 mg/kg bw per day; Butenhoff et al., 2012b). Hepatocellular hypertrophy accompanied by cytoplasmic vacuolation was also observed in monkeys, with a NOAEL and LOAEL of 0.15 and 0.75 mg/kg bw per day, respectively. Therefore, increased liver weight and hepatocellular hypertrophy are considered in the dose-response assessment-despite their potential to be adaptive, rather than adverse, effects-as a means of preventing the more serious histological effects observed in other studies or at higher doses. The NOAEL of 0.024 mg/kg bw per day was adjusted to a value of 0.021 mg/kg bw per day to account for decreased purity of the test material, which was only 86.9%.
The use of conservative endpoints for liver effects is not contradicted by epidemiology studies. Human studies demonstrated weak and inconsistent evidence of alterations of hepatic enzymes due to PFOS exposure, with positive, negative, and absent associations for hepatic parameters, depending on the study. Associations between changes in ALT levels of former perfluoroalkyl workers (3M plant) and geographically exposed individuals (included in the C8 project) and median PFOS serum levels ranging from 18 to 366 ng/mL have been reported (Gallo et al., 2012; Olsen et al., 2012); the ALT levels were increased in the environmentally-exposed population and decreased in the occupationally-exposed. No effect on liver enzyme levels was observed in a cross-sectional study of PFOS workers with serum levels in the range of 20-2,110 ng/mL (Olsen et al., 2003b).The serum values in the study with positive results are one to two orders of magnitude lower than the serum NOAEL and LOAEL values for the Butenhoff study (1,310 and 7,600 ng/mL, respectively).
Changes in thyroid hormone levels-which were observed in monkeys, rats, and mice-are also proposed as a critical effect for this analysis. The observed effects were typically decreases in T3 and T4 that were often observed at the lowest doses in studies. Corresponding increases in TSH were observed in a 26-week study of cynomolgus monkeys, but not in the rodent studies. Authors of a 26-week study of cynomolgus monkeys (Seacat et al., 2002) concluded that a LOAEL for thyroid hormone changes was 0.75 mg/kg bw per day; however, Health Canada (2013c) performed a statistical reanalysis of the data from Seacat et al. (2002) that allowed for improved interpretation of measures at multiple timepoints. The reanalysis identified dose effects for decreases in total T3 in both sexes and total T4 in females only, and dose-time effects for total T3, total T4, and TSH. The LOAEL and NOAEL values for changes in thyroid hormone levels as identified by the reanalysis were 0.15 and 0.03 mg/kg bw per day, respectively. Most rat studies had no NOAEL; the lowest LOAELs were in a similar range to the monkeys, as outlined in Section 9.2.2.4. As the NOAEL of 0.03 mg/kg bw per day from the 26-week monkey study is lower than the NOAEL for hepatocellular hypertrophy, thyroid hormone changes are considered as a potential critical effect for this assessment. Effects on thyroid hormone levels were not studied in many mouse studies, but decreases in T4 were observed only at higher levels (with the lowest LOAEL of 5 mg/kg bw per day in pups exposed in utero [Lau et al., 2003]). The chronic study of rats (Butenhoff et al., 2012b) did not identify any histological changes to the thyroid; however, the study did not explore changes in thyroid hormone levels, which can impact various systems in the body even in absence of structural changes to the thyroid. Epidemiology studies do not indicate any clear trends in PFOS-induced thyroid hormone changes-although some decreases in T3 and T4 were observed in association with increased PFOS levels, other studies indicated positive or absent associations.
Several of the studies identifying decreases in T3 and T4 levels were one-generation studies (primarily in rats), with observations of thyroid hormone level changes in both dams and pups. These thyroid hormones play an important role in development of fetal organs, including that of the central nervous system, with deficiencies observed to result in worsened neurobehavioural outcomes in animals and humans (Pop et al., 1999; Koibuchi and Chen, 2000; Morreale de Escobar, 2004; Williams, 2008; Delahunty et al., 2010; Gilbert et al., 2012; Schroeder and Privalsky, 2014). Several studies have provided some evidence of neurodevelopmental effects resulting from PFOS exposure (including changes in the brain ultrastructure, gene and protein expression, and learning abilities in rats, and motor behaviour in both rats and mice), with many LOAELs in a similar range to those observed for thyroid effects (see Section 9.2.5). In the one study (in rats) that investigated both thyroid and neurodevelopmental effects (Lau et al., 2003), lower T4 levels were observed in conjunction with small decreases in prefrontal cortex choline acetyltransferase levels (which is sensitive to thyroid hormone status), but no changes in learning and memory behaviours were noted. Although several studies indicate changes in motor behaviour in rats and mice at doses of 0.3-0.75 mg/kg bw per day (Johansson et al., 2008; Butenhoff et al., 2009; Onishchenko et al., 2011; Wang et al., 2015), weaknesses in each of the studies preclude their use as critical studies for these low doses. Study design affected the mouse studies, as Johansson et al. (2008) only provided the dose on a single day, and Onischenko et al. (2011) only contained a single dose group. The rat studies had inconsistent results. In Butenhoff et al. (2009), changes in motor activity were only observed on a single day, which was different in males and females (only at PND 17 in males exposed to 0.3 and 1 mg/kg bw per day, and only at PND 21 in females exposed to 1 mg/kg bw per day, with no effects in either sex at PND 13 or 61). In the Wang et al. (2015) study, rat pups were exposed in utero, lactationally, or both to 5 and 15 mg/L in drinking water (equivalent to 0.7 and 2.1 mg/kg bw per day using Health Canada's [1994] assumption of 1 ppm in water = 0.14 mg/kg bw per day in rats, which might not be relevant for pregnant or lactating dams). Effects on learning ability were observed in both dose groups, but more consistently at the high dose; at the low dose, changes were observed at fewer measurement days and were observed in the groups exposed only in utero or lactationally, and not in the group exposed over both periods. In cross-sectional epidemiological studies, although one study reported a positive association between increased PFOS levels and neurodevelopmental effects (reported ADHD), no clear relationships were observed for this endpoint in limited and equivoqual epidemiological evidence; no associations were observed for other neurodevelopmental milestones. If further studies demonstrate consistency of effects at low levels, the endpoint could be considered as a potential critical effect for PFOS exposures; in the absence of consistent effects at low levels, a TDI based on liver or thyroid effects is assumed to be sufficiently protective of neurobehavioural changes.
Changes in serum lipid levels were also observed around the levels at which liver and thyroid effects occur. Typical observed changes were decreases in total cholesterol, HDL, and triglycerides. The lowest dose at which serum lipid changes were observed was in the 26-week study of Cynomolgus monkeys, where decreases in HDL were observed at 0.03 mg/kg bw per day (i.e., the NOAEL for the previously-described thyroid effects). The lowest LOAEL for mice was 0.166 mg/kg bw per day (Fair et al., 2011), and for rats was 0.4 mg/kg bw per day (Luebker et al., 2005b). These effects are important for consideration during the assessment of PFOS risks, as epidemiology studies tend to demonstrate minor positive (albeit inconsistent, and of questionable clinical importance) associations between PFOS and serum cholesterol levels. Because inconsistencies in effect were observed between the two databases, and within the epidemiology database, and clear dose-response relationships were absent in the animal studies, quantitative assessments were not performed for serum lipid effects. Based on the present database, a TDI based on liver or serum effects is assumed to be sufficiently protective of lipid changes.
To reflect the large interspecies differences in pharmacokinetics, PODHEQ are calculated for both of the proposed critical effects, as follows:
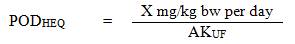
Figure 6 - Text Equivalent
- X mg/kg bw per day is the point-of-departure associated with the NOAEL for hepatocellular hypertrophy or thyroid hormone changes described above; and
- AKUF is the appropriate dose- and species-specific adjustment factor (as described in Section 8.6.2).
Using the calculated PODHEQ, the non-cancer TDIs are calculated as follows:
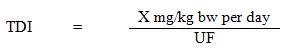
Figure 7 - Text Equivalent
- X mg/kg bw per day is the PODHEQ calculated for each critical effect, as described above; and
- UF is the composite uncertainty factor, as described below.
The PODHEQ and TDIs for each critical health effect are calculated and presented in Table 4 below:
| Hepatocellular hypertrophy | Thyroid hormone changes | |
|---|---|---|
| Study | Butenhoff et al., 2012b | Seacat et al., 2002 |
| NOAEL (mg/kg bw per day) | 0.021 | 0.03 |
| AKUF | 14 | 4 |
| PODHEQ (mg/kg bw per day) | 0.0015 | 0.0075 |
| Composite UF | 25 | 75 |
| TDI (mg/kg bw per day) | 0.00006 | 0.0001 |
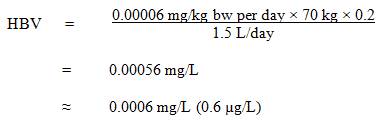
Figure 8 - Text Equivalent
- 0.00006 mg/kg bw per day is the TDI derived above;
- 70 kg is the average body weight of an adult;
- 0.2 is the default allocation factor for drinking water, used as a "floor value", since drinking water is not a major source of exposure and there is evidence of widespread presence in at least one of the other media (air, food, soil, or consumer products) (Krishnan and Carrier, 2013); and
- 1.5 L/day is the daily volume of water consumed by an adult; dermal and inhalation exposures from bathing and showering are not considered to be significant routes of exposure (as described in Section 5.7).
10.3 Comparison of cancer and non-cancer risk assessment
The HBV for the non-cancer assessment, which was 0.0006 mg/L using data of histological changes in rat liver, is more conservative than the HBV for hepatocellular tumours of 0.01 mg/L. The proposed HBV of 0.0006 mg/L that was derived for non-cancer effects is therefore considered to be sufficiently protective of the carcinogenic effects of PFOS.10.4 International considerations
A memorandum published by the U.S. EPA Office of Water indicates a Provisional Health Advisory (PHA) of 0.2 μg/L (0.0002 mg/L) for PFOS (U.S. EPA, 2009b). This PHA was derived from a NOAEL of 0.03 mg/kg bw per day for increased levels of TSH in males, reduced total T3 levels in males and females and reduced levels of high-density lipoproteins (HDL) in female Cynomolgus monkeys orally exposed to PFOS for 183 days (Seacat et al., 2002). An uncertainty factor of 390 (×10 for intraspecies differences, ×3 for interspecies toxicodynamic differences and ×13 for interspecies toxicokinetic differences) was applied, resulting in a reference dose rounded at 80 ng/kg bw per day (U.S. EPA, 2009b). This reference dose (RfD) was also indicated in a memorandum emitted by the U.S. EPA Office of Solid Waste and Emergency Response (U.S. EPA, 2009c). Based on U.S. EPA policy, the PHA (0.2 μg/L) was further derived for a 10-kg child consuming 1 L water per day using a default assumption that drinking water contributes 20% of total exposure to PFOS (U.S. EPA, 2009b).The Minnesota Department of Health (MDH, 2008c; 2008d) derived a Health Risk Limit (HRL) of 0.3 μg/L (0.0003 mg/L). This value was based on the decreased HDL cholesterol, decreased T3 and increased TSH observed in monkeys exposed to ≥ 0.03 mg/kg bw per day (Seacat et al., 2002; Thomford, 2002). More precisely, the benchmark dose (BMDL; level of response not specified) for these effects was estimated at 35,000 ng/mL PFOS in monkey serum. A human equivalent dose (HED) of 2,500 ng/kg bw per day (2.5 μg/kg bw per day, or 0.0025 mg/kg bw per day) was calculated based on the elimination of PFOS in humans (using human half-life data to estimate clearance). An uncertainty factor of 30 (×10 for human variability and ×3 for interspecies toxicodynamic differences) was applied to the HED, resulting in a reference dose rounded at 80 ng/kg bw per day (0.08 μg/kg bw per day, or 0.00008 mg/kg bw per day). Steady-state uptake/elimination was estimated to occur after 27 years of exposure, therefore a time-weighted 95th percentile intake for the first 27 years of life (0.049 L/kg bw per day) was used as the daily water ingestion. This, along with a relative source contribution of 20%, resulted in a Health Risk Limit (HRL) rounded at 0.3 μg/L (MDH, 2008c; MDH, 2008d).
A drinking water guideline of 0.3 μg/L (0.0003 mg/L) was derived by the UK Health Protection Agency (UK HPA, 2007; 2009) based on a TDI of 300 ng/kg bw per day (0.3 μg/kg bw per day) previously derived by the UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (UK COT, 2006). This TDI was derived from a point‑of-departure (0.03 mg/kg bw per day) corresponding to the NOAEL for decreased serum T3 levels in monkeys exposed for 26 weeks (Thomford, 2002; Seacat et al., 2002), divided by an uncertainty factor of 100 (for intra- and inter-species variation). The drinking water guideline (0.3 μg/L) was derived from this TDI, using an allocation factor of 10%, a body weight of 10 kg and a water ingestion rate of 1 L per day for a one-year-old child (UK HPA, 2007).
In their scientific opinion document on contaminants in the food chain, the CONTAM panel under the European Food Safety Authority derived a TDI of 150 ng/kg bw per day (0.15 μg/kg bw per day) based on a NOAEL of 0.03 mg/kg bw per day (associated with a plasma concentration of 13,200 ng/mL in females)(EFSA, 2008). This NOAEL was taken from an oral dietary study in Cynomolgus monkeys; changes in lipids and thyroid hormones were observed at the next higher dose (0.15 mg/kg bw per day) (Seacat et al., 2002). The NOAEL was divided by an uncertainty factor of 200 (×10 for interspecies differences, ×10 for intraspecies differences and ×2 to compensate for uncertainties in connection to the relatively short duration of the key study and the internal dose kinetics).
11.0 Rationale
PFOS is an anthropogenic compound which was used for water, oil and/or stain resistance on surface and paper-based applications, such as rugs and carpets, fabric and upholstery. It was also used in specialized chemical applications, such as fire-fighting foams, hydraulic fluids, and carpet spot removers. PFOS is no longer manufactured, imported, sold, offered for sale or used in Canada, unless incidentally present, with certain exemptions and under certain conditions (e.g., aviation hydraulic fluids). Because of its extremely persistent nature, PFOS can still be found in the environment. Canadians can be exposed to PFOS in food, consumer products, dust, and drinking water. The major sources of PFOS is expected to be food and consumer products, however, the proportion of exposure from drinking water can increase in individuals living in areas with contaminated drinking water. Based on its physico-chemical properties, exposure to PFOS via inhalation and dermal routes during showering or bathing is expected to be negligible.The carcinogenicity of PFOS has not been evaluated by IARC. Chronic exposure to PFOS has been associated with both cancer and non-cancer effects in animals and humans. HBVs for both endpoints have been calculated, with the non-cancer effects resulting in a lower, more conservative HBV.
Epidemiological studies have shown associations between exposure to PFOS and multiple non-cancer health outcomes, such as reproductive, developmental, and immunological effects. However, these studies cannot be used to derive the non-cancer HBV for PFOS due to their limitations, including in terms of study design, bias and confounders. In animals, non-cancer effects observed at the lowest levels of exposure include immunological effects, liver effects, effects on the thyroid and changes in serum lipid levels. For various reasons described in section 10.2, the most appropriate endpoint to derive a HBV for PFOS is hepatocellular hypertrophy (liver effects) in rats, supported quantitatively by the estimated value for thyroid effects in monkeys. Using a TDI approach, a HBV of 0.0006 mg/L (0.6 µg/L) has been calculated for the non-cancer effects of PFOS based on liver effects in rats. This HBV is considered to be sufficiently protective of both cancer and non-cancer effects of PFOS.
A MAC of 0.0006 mg/L (0.6 µg/L) is proposed for PFOS in drinking water. The proposed MAC for PFOS can be measured by available analytical methods and is achievable by municipal and residential treatment technologies. As part of its ongoing guideline review process, Health Canada will continue to monitor new research and recommend any change to the guideline that is deemed necessary.
12.0 References
3M Company (1996). Determination of physico-chemical properties of sample D-1. AR226-0973. [As cited in Martin et al. (2010)].3M Company (1999). The science of organic fluorochemistry. Letter of Frank D. Kover, Chief, Chemical Information and Testing Branch, Chemical Control Division, Office of Pollution Prevention and Toxics, U.S. EPA. Available at: www.fluoridealert.org/wp-content/pesticides/pfos.fr.final.docket.0006.pdf
3M Company (2001). Soil adsorption/desorption study of potassium perfluorooctanesulfonate (PFOS). Laboratory project number E00-1311. EPA Docket AR226-1030a030. 3M Environmental Laboratory, St. Paul, Minnesota. [As cited in Beach et al. (2006)].
3M Company (2008). Interim report #17-Analysis of Woodbury waste site water samples. Maplewood, Minnesota.
Abbott, B.D., Wolf, C.J., Das, K.P., Zehr, R.D., Schmid, J.E., Lindstrom, A.B., et al. (2009). Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR alpha) in the mouse. Reprod Toxicol, 27(3-4): 258-265.
Abe , T., Baba, H., Itoh, E. and Tanaka, K. (2001). Separation of perfluoroalkylsulfinic acids and perfluoroalkylsulfonic acids by ion-exclusion chromatioography. J. Chromatogr. A, 920:173-180.
Ahrens, L. (2011). Polyfluoroalkyl compounds in the aquatic environment: a review of their occurrence and fate. J. Environ. Monit., 13(1): 20-31.
Alberta Environment and Water (2013). Personal communication from Dr. Donald Reid.
Alexander, B.H. and Olsen, G.W. (2007). Bladder cancer in perfluorooctanesulfonyl fluoride manufacturing workers. Ann. Epidemiol., 17(6): 471-478.
Alexander, B.H., Olsen, G.W., Burris, J.M., Mandel, J.H. and Mandel, J.S. (2003). Mortality of employees of a perfluorooctanesulphonyl fluoride manufacturing facility. Occup. Environ. Med., 60(10): 722-729.
Andersen, C.S., Fei, C., Gamborg, M., Nohr, E.A., Sørensen, T.I. and Olsen, J. (2010). Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am. J. Epidemiol., 172(11): 1230-1237. Erratum in: Am. J. Epidemiol., 173(12):1475.
Andersen, M.E., Clewell, H.J., 3rd, Tan, Y.M., Butenhoff, J.L. and Olsen, G.W. (2006). Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys-probing the determinants of long plasma half-lives. Toxicology, 227(1-2): 156-164.
Apelberg, B.J., Witter, F.R., Herbstman, J.B., Calafat, A.M., Halden, R.U., Needham, L.L. et al. (2007). Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect, 115(11): 1670-1676.
Appleman, T.D., Dickenson, E.R.V., Bellona, C. and Higgins, C.P. (2013). Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. J. Hazard. Mater., 260(15): 740-746.
Appleman, T.D., Higgins, C.P., Quiñones, O., Vanderford, B.J., Kolstad, C., Zeigler-Holady, J.C. and Dickenson, E.R.V. (2014). Treatment of poly- and perfluoroalkyl substances in U.S. full-scale water treatment systems. Water Res. 51:246-255.
Arbuckle, T., Kubwabo, C., Walker, M., Davis, K., Lalonde, K. Kosarac, I., Wen, S. and Arnold, D. (2013). Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int. J. Hyg. Envir. Health, 216:184-194.
Arsenault, G., Chittim, B., McAlees, A., McCrindle, R., Riddell, N. and Yeo, B. (2008). Some issue relating to the use of perfluorooctane sulfonate (PFOS) samples as reference standard. Chemosphere, 70:620-625.
Atkinson, C., Blake, S., Hall, T., Kanda, R. and Rumsby, P. (2008). Survey of the prevalence of perfluorooctane sulphonate (PFOS), perfluorooctanoic acid (PFOA) and related compounds in drinking water and their sources. WRc Ref: DEFRA 7585, February. Available at: http://dwi.defra.gov.uk/research/completed-research/reports/DWI70_2_212PFOS.pdf.
ATSDR (2009). Toxicological profile for perfluoroalkyls, draft for public comment. Agency for Toxic Substances and Disease Registry Available at: www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1117&tid=237
Austin, M.E., Kasturi, B.S., Barber, M., Kannan, K., MohanKumar, P.S. and MohanKumar, S.M. (2003). Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ Health Perspect, 111(12): 1485-1489.
Awad, E., Zhang, X., Bhavsar, S.P., Petro, S., Crozier, P.W., Reiner, E.J., Fletcher, R., Tittlemier, S.A. and Braekevelt, E. (2011). Long-term environmental fate of perfluorinated compounds after accidental release at Toronto airport. Environ. Sci. Technol., 45(19): 8081-8089.
AWWA. (2011). Water quality and treatment, a handbook of community water supplies. Sixth Edition. James K. Edzwald, Editor. American Water Works Association and McGraw-Hill. Denver, Colorado.
Backe, W, Thomas, J, Day, C and Field, J.A. (2013). Zwitterionic, cationic, and anionic fluorinated chemicals in aqueous film forming foam formulations and groundwater from U.S. military bases by non-aqueous large-volume injection HPLCMS/ MS. Environ. Sci. Technol., 47 (10): 5226-5234.
Baduel, C., Paxman, C.J. and Mueller J.F. (2015). Perfluoroalkyl substances in a firefighting training ground (FTG), distribution and potential future release. J. Hazard. Mater., 296: 46-53.
Bannasch, P. (2003). Comments on R. Karbe and R.L. Kerlin (2002). Cystic degeneration/spongiosis hepatis (Toxicol. Pathol., 30(2), 216-227). Toxicol. Pathol., 31: 566-570.
Bannasch, P. and Zerban, H. (1997). Spongiosis hepatis and spongiotic pericytoma, rat. In: Jones, T.C., Popp, J.A. and Mohr, U. (eds.) Digestive System. Second Edition. Monographs on Pathology of Laboratory Animals. Springer-Verlag, Berlin.
Beach, S.A., Newsted, J.L., Coady, K. and Giesy, J.P. (2006). Ecotoxicological evaluation of Perfluorooctanesulfonate (PFOS). Rev. Environ. Contam. Toxicol., 186: 133-174.
Benskin, J.P., De Silva, A.O., Martin, L.J., Arsenault, G., McCrindle, R., Riddell, N. et al. (2009). Disposition of perfluorinated acid isomers in Sprague-Dawley rats; part 1: single dose. Environ. Toxicol. Chem., 28(3):542-554.
Benskin, J.P., De Silva, A.O. and Martin, J.W. (2010), Isomer profiling of perfluorinated substances as a tool for source tracking: a review of early findings and future application. Reviews of Environmental Contamination and Toxicology. P. de Voogt (ed.). Springer Science+Business Media, LLC.
Berger, U., Kaiser, M., Karrman, A., Barber, J. and van Leeuwen, S. (2011). Recent developments in trace analysis of poly-and perfluoroalkyl substances. Anal. Bional. Chem., 4000:1625-1635.
Berger, U., Langlois, I., Oehme, M. and Kallenborn, R. (2004). Comparison of three types of mass spectrometer for high-performance liquid chromatography/mass spectrometry analysis of perfluoroalkylated substances and fluorotelomer alcohol. Eur. J. Mass Spectrom. 10:579-588.
Berryman, D., Salhi, C., Bolduc, A., Deblois , C. and Tremblay, H. (2012). Les composés perfluorés dans les cours d'eau et l'eau potable du Québec méridional. Québec, Ministère du Développement durable, de l'Environnement, de la Faune et des Parcs, Direction du suivi de l'état de l'environnement.
Biesemeier, J.A. and Harris, D.L. (1974). Eye and skin irritation report on sample T-1117. Report. Project No. 4102871, WARF Institute Inc. [As cited in OECD (2002); EFSA (2008)].
Bio/Dynamics, Inc. (1979). An acute inhalation toxicity study of T-2306 CoC in the rat. # 78-7185. [As cited in Health Canada (2006)].
Bloom, M.S., Kannan, K., Spliethoff, H.M., Tao, L., Aldous, K.M. and Vena, J.E. (2010). Exploratory assessment of perfluorinated compounds and human thyroid function. Physiol. Behav., 99(2): 240-245.
Bogdanska, J., Borg, D., Sundström, M., Bergström, U., Halldin, K., Abedi-Valugerdi, M., Bergman, A., Nelson, B., Depierre, J. and Nobel, S. (2011). Tissue distribution of 35S-labelled perfluorooctane sulfonate in adult mice after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology, 284(1-3):54-62.
Bonefeld-Jorgensen, E., Long, M., Bossi, R., Ayotte, P., Asmund, G., Kruger, T., et al. (2011). Perfluorinated compounds are related to breast cancer risk in greenlandic inuit: A case control study. Environ. Health, 10(1): 88. Available at: www.ehjournal.net/content/10/1/88.
Borg, D., Bogdanska, J., Sundström, M., Nobel, S., Hakansson, H., Bergman, et al. (2010). Tissue distribution of (35)S-labelled perfluorooctane sulfonate (PFOS) in C57Bl/6 mice following late gestational exposure. Reprod Toxicol, 30(4): 558-65.
Boulanger, B., Peck, A.M., Schnoor, J.L. and Hornbuckle, K.C. (2005). Mass budget of perfluorooctane surfactants in Lake Ontario. Environ Sci Technol, 39: 74-79. As cited in EFSA (2008).
Boyd, G., Tucillo, M.E., Sandvig, A., Pelaez, M., Han, C. and Dionysious, D.D. (2013). Nanomaterials: Removal processes and beneficial applications in treatment. J. Am. Water Works Assoc.,105(12): 699-708.
Brooke, D., Footitt, A. and Nwaogu, T.A. (2004). Environmental risk evaluation report: Perfluorooctanesulphonate (PFOS). Available at: www.pops.int/documents/meetings/poprc/submissions/Comments_2006/sia/pfos.uk.risk.eval.report.2004.pdf
Buck, R. C., Franklin, J., Berger, U., Conder, J. M., Cousins, I. T., de Voogt, P., Jensen, A. A.,Kannan, K., Mabury, S. A. and van Leeuwen, S. P. J. (2011). Perfluoroalkyl and polyfluoroalkylsubstances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag., 7(4), 513-541.
Butenhoff, J.L., Ehresman, D.J., Chang, S.C., Parker, G.A. and Stump, D.G. (2009). Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: developmental neurotoxicity. Reprod Toxicol., 27(3-4): 319-330.
Butenhoff, J.L., Pieterman, E., Ehresman, D.J., Gorman, G.S., Olsen, G.W., Chang, S.C. and Princen, H.M. (2012a). Distribution of perfluorooctanesulfonate and perfluorooctanoate into human plasma lipoprotein fractions. Toxicol. Lett., 210(3):360-365.
Butenhoff, J.L., Chang, S.C., Olsen, G.W. and Thomford, P.J. (2012b). Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology, 293(1-3): 1-15.
Butt, C.M., Berger, U., Bossi, R. and Tomy, G.T. (2010). Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci. Total Environ., 408(15): 2936-2965.
C8 Science Panel website. www.c8sciencepanel.org/panel.html.
Campbell, J.L., Jr. and Clewell, H.J., III. (2013). Report on the perfluorooctanesulfonic acid (PFOS) kinetic models and dosimetry. Final contract report to Health Canada.
Cao, M.H., Wang, B.B., Yu, H.S., Wang, L.L. , Yuan, S.H. , and Chen, J. (2010). Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation. J. Hazard. Mater., 179:1143-1146.
Carter, K.E. and Farrell, J. (2010). Removal of perfluorooctane and perfluorobutane sulfonate from water via carbon adsorption and ion exchange. Sep. Sci. Technol., 45:762-767.
Case, M.T., York, R.G. and Christian, M.S. (2001). Rat and rabbit oral developmental toxicology studies with two perfluorinated compounds. Int. J. Toxicol., 20(2): 101-109.[ As cited in EFSA (2008)].
Chan, E., Burstyn, I., Cherry, N., Bamforth, F. and Martin, J.W. (2011). Perfluorinated acids and hypothyroxinemia in pregnant women. Environ. Res., 111(4): 559-564.
Chang, S.C., Ehresman, D.J., Bjork, J.A., Wallace, K.B., Parker, G.A., Stump, D.G. and Butenhoff, J.L. (2009). Gestational and lactational exposure to potassium perfluorooctanesulfonate (K+PFOS) in rats: toxicokinetics, thyroid hormone status, and related gene expression. Reprod. Toxicol., 27(3-4):387-399.
Chang, S.C., Noker, P.E., Gorman, G.S., Gibson, S.J., Hart, J.A., Ehresman, D.J. and Butenhoff, J.L. (2012). Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod. Toxicol., 33(4):428-440.
Château-Degat, M.L., Pereg, D., Dallaire, R., Ayotte, P., Dery, S. and Dewailly, E. (2010). Effects of perfluorooctanesulfonate exposure on plasma lipid levels in the Inuit population of Nunavik (Northern Quebec). Environl Resl, 110(7): 710-717.
Chen, J. and Zhang, P. (2006). Photodegradation of perfluorooctanoic acid in water under irradiation of 254 nm and 185 nm light by use of persulfate. Water Sci. Technol., 54(11-12):317-325.
Chen, J., Zhang, P-Y. and Liu, J. (2007). Photodegradation of perfluorooctanoic acid by 185 nm vacuum ultraviolet light. J. Environ. Sci., 19:387-390.
Chen, T., Zhang, L., Yue, J.Q., Lv, Z.Q., Xia, W., Wan, Y.J., et al. (2012). Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reprod Toxicol., 33(4): 538-45.
Christensen, K.Y., Maisonet, M., Rubin, C., Holmes, A., Calafat, A.M., Kato, K. et al. (2011). Exposure to polyfluoroalkyl chemicals during pregnancy is not associated with offspring age at menarche in a contemporary British cohort. Environ. Int., 37(1): 129-135.
Christian, M.S., Hoberman, A.M. and York, R.G. (1999). combined oral (gavage) fertility, developmental and perinatal/postnatal reproduction toxicity study of pfos in rats. Sponsor Study No. 6295-.9. Protocol number 418-008. Argus Research Laboratories, Inc., Horsham, PA U.S EPA. Docket 8EHQ-0200-00374. (also cited as Argus Research Laboratories, Inc.). [As cited in OECD (2002); Health Canada (2006); EFSA (2008)].
Chularueangaksorn, P., Tanaka, S., Fujii, S. and Kunacheva, C. (2013). Adsorption of perfluorooctanoic acid (PFOA) onto anion exchange resin, non-ion exchange resin, and granular activated carbon by batch and column. Desalination Water Treat., 52:6542-6548.
Chularueangaksorn, P., Tanaka, S., Fujii, S., Kunacheva, C. (2014). Batch and column adsorption of perfluorooctane sulfonate on anion exchange resins and granular activated carbon. J. Appl. Polym. Sci., 131:39782-39788.
Clarke, B.O. and Smith, S.R. (2011). Review of 'emerging' organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int., 37(1): 226-247.
Corning Hazleton Inc. (1997). Final report: Primary eye irritation/corrosion study of T-6684 in rabbits (OECD Guidelines). # 61101151. [As cited in Health Canada (2006)].
Corton, J.C., Cunningham, M.L., Hummer, B.T., Lau, C., Meek, B., Peters, J.M., Popp, J.A., Rhomberg, L., Seed, J. and Klaunig, J.E. (2014). Mode of action framework analysis for receptor-mediated toxicity: the peroxisome proliferator-activated receptor alpha (PPARα) as a case study. Crit. Rev. Toxicol., 44(1):1-49.
Crump, K.S. (1984). A new method for determining allowable daily intakes. Fundam. Appl. Toxicol., 4: 854-871.
Cui, L., Zhou, Q.F., Liao, C.Y., Fu, J.J. and Jiang, G.B. (2009). Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch. Environ. Contam. Toxicol., 56(2): 338-349.
Cui, L., Liao, C.Y., Zhou, Q.F., Xia, T.M., Yun, Z.J. and Jiang, G.B. (2010). Excretion of PFOA and PFOS in male rats during a subchronic exposure. Arch. Environ. Contam. Toxicol., 58(1): 205-13.
Dai, Y., Niu, J., Yin, L., Xu, J. and Sun, K. (2013). Enhanced sorption of perfluorooctane sulfonate (PFOS) on carbon nanotube-filled electrospunnanofibrous membranes. Chemosphere, 93(8):1593-1599.
Dallaire, R., Dewailly, E., Pereg, D., Dery, S. and Ayotte, P. (2009). Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ. Health Perspect., 117(9):1380-1386.
De Silva, A.O., Benskin, J.P., Martin, L.J., Arsenault, G., McCrindle, R., Riddell, N., Martin, J.W. and Mabury, S.A. (2009). Disposition of perfluorinated acid isomers in Sprague-Dawley rats; Part 2: Subchronic dose. Environ. Toxicol. Chem., 28(3): 555-567.
Dean, W.P. and Jessup, D.C. (1978). Acute oral toxicity (LD50) study in rats. International Research and Development Corporation, Study No. 137-091, May 5, 1978. U.S. Environmental Protection Agency Administrative Record 226-0419. [As cited in OECD (2008)].
Delahunty, C., Falconer, S., Hume, R., Jackson, L., Midgley, P., Mirfield, M., Ogston, S., Perra, O., Simpson, J., Watson, J., Willatts, P., Williams, F. and the Scottish Preterm Thyroid Group. (2010). Levels of neonatal thyroid hormone in preterm infants and neurodevelopmental outcome at 5 1/2 years: millennium cohort study. J. Clin. Endocrinol. Metab., 95(11): 4898-4908.
Deng, S., Yu, Q., Huang, J., Yu, G. (2010). Removal of perfluorooctane sulfonate from wastewater by anion exchange resins: Effects of resin properties and solution chemistry. Water Res. 44(18):5188-5195.
Deng, S., Zhou, Q., Yu, G., Huang, J. and Fan, Q. (2011). Removal of perfluorooctanoate from surface water by polyaluminium chloride coagulation. Water Res., 45:1774-1780.
Deng, S., Zhang, Q., Nie, Y., Wei, H., Wang, B., Huang, J., Yu, G., Xing, B., (2012). Sorption mechanisms of perfluorinated compounds on carbon nanotubes. Environ. Pollut. 168:138-144.
Dickenson, E.R.V. and Higgins, C. (2013). The removal of perfluoroalklyl and polyfluoroalkly substances by North American water treatment practices. Water Research Foundation, Denver, Colorado. Draft Report. (in press)
Dillert, R., Bahnemann, D. and Hidaka, H.(2007). Light-induced degradation of perfluorocarboxylic acids in the presence of titanium dioxide. Chemosphere, 67:785-792.
Dolman, S. and Pelzing, M. (2011). An optimized method for the determination of perfluorooctanoic acid, perfluorooctane sulfonate and other perfluorochemicals in different matrices using liquid chromatography/ion-trap mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 879(22): 2043-2050.
Dong, G.H., Zhang, Y.H., Zheng, L., Liu, W., Jin, Y.H. and He, Q.C. (2009). Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch Toxicol., 83(9): 805-815.
Dong, G.H., Liu, M.M., Wang, D., Zheng, L., Liang, Z.F. and Jin, Y.H. (2011). Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch Toxicol., 85(10): 1235-1244.
Dong, G.H., Wang, J., Zhang, Y.H., Liu, M.M., Wang, D., Zheng, L. et al. (2012a). Induction of p53-mediated apoptosis in splenocytes and thymocytes of C57BL/6 mice exposed to perfluorooctane sulfonate (PFOS). Toxicol. Appl. Pharmacol., 264(2): 292-299.
Dong, G.H., Zhang, Y.H., Zheng, L., Liang, Z.F., Jin, Y.H. and He, Q.C. (2012b). Subchronic effects of perfluorooctanesulfonate exposure on inflammation in adult male C57BL/6 mice. Environ. Toxicol., 27(5): 285-296.
Dong, G.H., Tung, K.Y., Tsai, C.H., Liu, M.M., Wang, D., Liu, W. et al. (2013). Serum polyfluoroalkyl concentrations, asthma outcomes, and immunological markers in a case-control study of taiwanese children. Environ. Health Perspect., 121(4): 507-513.
Dudley, L.-A., Lindstrom, A., Strynar, M., McMillan, L. and Knappe, D. (2012). Removal of perfluorinated compounds by powdered activated carbon: effects of adsorbent and background water characteristics. Presented at 2012 AWWA Annual Conference in Dallas, TX, June 10 -14, 2012. 2012 Annual Conference Proceedings.American Water Works Association, Catalog No. ACE_0076609.
EFSA (2008). Opinion of the scientific panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. European Food Safety Authority. EFSA Journal 653: 1-131. Available at: www.efsa.europa.eu/en/efsajournal/pub/653.htm
Elcombe, C.R., Elcombe, B.M., Foster, J.R., Chang, S.C., Ehresman, D.J., Noker, P.E., et al. (2012a). Evaluation of hepatic and thyroid responses in male Sprague Dawley rats for up to eighty-four days following seven days of dietary exposure to potassium perfluorooctanesulfonate. Toxicology, 293(1-3): 30-40.
Elcombe, C.R., Elcombe, B.M., Foster, J.R., Chang, S.C., Ehresman, D.J. and Butenhoff, J.L. (2012b). Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARα and CAR/PXR. Toxicology, 293(1-3): 16-29.
Emmett, E.A., Shofer, F.S., Zhang, H., Freeman, D., Desai, C. and Shaw, L.M. (2006). Community exposure to perfluorooctanoate: Relationships between serum concentrations and exposure sources. J. Occup. Environ. Med., 48: 759-770.
Environment Canada and Health Canada (2012). Proposed risk management approach for perfluorooctanoic acid (PFOA), its salts, and its precursors and long-chain (C9-C20) perfluorocarboxylic acids (PFCAs), their salts, and their precursors. Available at: www.ec.gc.ca/ese-ees/default.asp?lang=En&n=451C95ED-1.
Era, S., Harada, K.H., Toyoshima, M., Inoue, K., Minata, M., Saito, N., et al. (2009). Cleft palate caused by perfluorooctane sulfonate is caused mainly by extrinsic factors. Toxicology, 256(1-2): 42-47.
Eriksen, K.T., Sørensen, M., McLaughlin, J.K., Lipworth, L., Tjønneland, A., Overvad, K. et al. (2009). Perfluorooctanoate and perfluorooctanesulfonate plasma levels and risk of cancer in the general Danish population. J. Natl. Cancer Inst., 101(8): 605-609.
Eriksen, K.T., Raaschou-Nielsen, O., Sørensen, M., Roursgaard, M., Loft, S. and Møller, P. (2010). Genotoxic potential of the perfluorinated chemicals PFOA, PFOS, PFBS, PFNA and PFHxA in human HepG2 cells. Mutat.. Res., 700(1-2): 39-43.
Eschauzier, C., Beerendonk, E., Scholte-Veenendaal, P. and De Voogt, P. (2012). Impact of treatment processes on the removal of perfluoroalkyl acids from the drinking water production chain. Environ. Sci. Technol., 46 (3):1708-1715.
Fair, P.A., Driscoll, E., Mollenhauer, M.A., Bradshaw, S.G., Yun, S.H., Kannan, K., et al. (2011). Effects of environmentally-relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B6C3F1 mice. J. Immunotoxicol., 8(1): 17-29.
Fasano, W.J., Kennedy, G.L., Szostek, B., Farrar, D.G., Ward, R.J., Haroun, L. et al. (2005). Penetration of ammonium perfluorooctanoate through rat and human skin in vitro. Drug. Chem. Toxicol 28(1):79-90. [As cited in ATSDR (2009).]
Fei, C. and Olsen, J. (2011). Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ. Health Perspect., 119(4): 573-578.
Fei, C., McLaughlin, J.K., Tarone, R.E. and Olsen, J. (2007). Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect., 115(11): 16777-16782.
Fei, C., McLaughlin, J.K., Lipworth, L. and Olsen, J. (2008a). Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ. Health Perspect., 116(10): 1391-1395.
Fei, C., McLaughlin, J.K., Tarone, R.E. and Olsen, J. (2008b). Fetal growth indicators and perfluorinated chemicals: a study in the Danish National Birth Cohort. Am. J. Epidemiol., 168(1): 66-72.
Fei, C., McLaughlin, J.K., Lipworth, L. and Olsen, J. (2009). Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod., 24(5): 1200-1205.
Fei, C., McLaughlin, J.K., Lipworth, L. and Olsen, J. (2010a). Prenatal exposure to PFOA and PFOS and risk of hospitalization for infectious diseases in early childhood. Environ. Res., 110: 773-777.
Fei, C., McLaughlin, J.K., Lipworth, L. and Olsen, J. (2010b). Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand. J. Work Environ. Health, 36(5): 413-421.
Fitz-Simon, N., Fletcher, T., Luster, M.I., Steenland, K., Calafat, A.M., Kato, K. et al. (2013). Reductions in serum lipids with a 4-year decline in serum perfluorooctanoic acid and perfluorooctanesulfonic acid. Epidemiology, 24(4): 569-576. Erratum in: Epidemiology, 24(6):941.
Florentin, A., Deblonde, T., Diguio, N., Hautemaniere, A. and Hartemann, P. (2011). Impacts of two perfluorinated compounds (PFOS and PFOA) on human hepatoma cells: cytotoxicity but no genotoxicity? Int. J. Hyg. Environ. Health., 214(6): 493-499.
Flores, C., Ventura, F., Martin-Alonso, J. and Caixach, J. (2013). Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in N.E. Spanish surface water and their removal in a drinking water treatment plant that combines conventional and advanced treatment in parallel lines. Sci. Tot. Environ. 461: 618-626.
Franko, J., Meade, B.J., Frasch, H.F., Barbero, A.M. and Anderson, S.E. (2012). Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J. Toxicol. Environ. Health A., 75(1): 50-62
Fraser, A.J., Webster, T.F., Watkins, D.J., Nelson, J.W., Stapleton, H.M., Calafat, A.M. et al. (2012). Polyfluorinated compounds in serum linked to indoor air in office environments. Environ. Sci. Technol.., 46(2): 1209-1215.
Frisbee, S.J., Brooks, A.P., Jr., Maher, A., Flensborg, P., Arnold, S., Fletcher, T. et al. (2009). The C8 health project: design, methods, and participants. Environ. Health Perspect, 117(12): 1873-1882.
Frisbee, S.J., Shankar, A., Knox, S.S., Steenland, K., Savitz, D.A., Fletcher, T. et al. (2010). Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch. Pediatr. Adolesc. Med., 164(9): 860-869.
Fromme, H., Tittlemier, S.A., Völkel, W., Wilhelm, M. and Twardella, D. (2009). Perfluorinated compounds-exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health, 212(3): 239-270.
Fuentes, S., Colomina, M.T., Vicens, P. and Domingo, J.L. (2007a). Influence of maternal restraint stress on the long-lasting effects induced by prenatal exposure to perfluorooctane sulfonate (PFOS) in mice. Toxicol. Lett., 171(3): 162-170.
Fuentes, S., Colomina, M.T., Vicens, P., Franco-Pons, N. and Domingo, J.L. (2007b). Concurrent exposure to perfluorooctane sulfonate and restraint stress during pregnancy in mice: effects on postnatal development and behavior of the offspring. Toxicol. Sci., 98(2): 589-598.
Fuentes, S., Vicens, P., Colomina, M.T. and Domingo, J.L. (2007c). Behavioral effects in adult mice exposed to perfluorooctane sulfonate (PFOS). Toxicology., 242(1-3): 123-129.
Fujii, S., Polprasert, C., Tanaka, S. and Lien, N.P.H. (2007). New POPs in the water environment: distribution, bioaccumulation and treatment of perfluorinated compounds - a review paper. J. Water Supply Res. T, 56(5): 313-326.
Furdui, V., Crozier, P., Reiner, E. and Mabury, S. (2008). Trace level determination of perfluorinated compounds in water by direct injection. Chemosphere 73:S24-S530.
Gallo, V., Leonardi, G., Genser, B., Lopez-Espinosa, M.J., Frisbee, S.J., Karlsson, L. et al. (2012). Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ. Health Perspect, 120(5): 655-660.
Gilbert, M.E., Rovet, J., Chen, Z. and Koibuchi, N. (2012). Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology, 33: 842-852.
Giri, R.R., Ozaki, H., Morigaki, T., Taniguchi, S. and Takanami, R. (2011). UV photolysis of perfluorooctanoic acid (PFOA) in dilute aqueous solution. Water Sci. Technol., 63(2): 276-282.
Giri, R.R, Ozaki, H., Okada, T., Taniguchi, S. and Takanami, R. (2012). Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chemi. Eng. J., 180: 197-203.
Giri, R. R., Ozaki, H., Guo, X., Takanami, R. and Taniguchi, S. (2013). Oxidative-reductive photodecomposition of perfluorooctanoic acid in water. Int. J. Environ. Sci. Te., DOI 10.1007/s13762-013-0312-2.
Goldenthal, E.I., Jessup, D.C., Geil, R.G. and Mehring, J.S. (1978a). Ninety-day subacute rat toxicity study. Study No. 137-085. International Research and Development Corporation. [As cited in OECD (2002); EFSA (2008); Health Canada (2012)].
Goldenthal, E.I., Jessup, D.C., Geil, R.G. and Mehring, J.S. (1978b). Ninety-day subacute rhesus monkey toxicity study. Study No. 137-092. International Research and Development Corporation. [As cited in OECD (2002); EFSA (2008); Health Canada (2012)].
González-Barreiro, C., Elena Martínez-Carballo, E., Sitka, A., Scharf, S. and Gans, O., (2006). Method optimization for determination of selected perfluorinated alkylated substances in water samples. Anal. Bioanal. Chem., 386:2123-2132.
Gortner, E.G. (1980). Oral teratology study of FC-95 in rats. Safety Evaluation Laboratory and Riker Laboratories, Inc. Experiment Number: 0680TR0008, December. [As cited in OECD (2002); EFSA (2008); Health Canada (2012)].
Government of Canada (2008). Perfluorooctane sulfonate and its salts and certain other compounds regulations. SOR/2008-178. Available at: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2008-178/page-1.html.
Government of Canada (2009). Regulations adding perfluorooctane sulfonate and its salts to the virtual elimination list. SOR/2009-15. Available at: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2009-15/page-1.html.
Grandjean, P. and Budtz-Jørgensen, E. (2013). Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environ. Health, 12:35.
Grandjean, P., Andersen, E.W., Budtz-Jørgensen, E., Nielsen, F., Mølbak, K., Weihe, P., Heilmann, C. (2012). Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA, 307(4): 391-397. Comment in: JAMA, 307(18):1910; author reply 1910-1. Erratum in: JAMA, 307(11):1142.
Granum, B., Haug, L.S., Namork, E., Stølevik, S.B., Thomsen, C., Aaberge, I.S., van Loveren, H., Løvik, M. and Nygaard, U.C. (2013). Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol., 10(4): 373-379.
Grasty, R.C., Bjork, J.A., Wallace, K.B., Wolf, D.C., Lau, C.S. and Rogers, J.M. (2005). Effects of prenatal perfluorooctane sulfonate (PFOS) exposure on lung maturation in the perinatal rat. Birth Defects Res. B Dev. Reprod. Toxicol., 74(5): 405-416. Erratum in: Birth Defects Res. B Dev. Reprod. Toxicol., 77(1):87: Wolf, DC [added].
Grasty, R.C., Grey, B.E., Lau, C.S. and Rogers, J.M. (2003). Window of susceptibility to perfluoroctane sulfonate (PFOS)-induced neonatal mortality in the rat. Birth Defects Res. B Dev. Reprod. Toxicol., 67(5): 315. Meeting abstract.
Greiner, A. and Wendorff, J. (2007). Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed., 46: 5670-5703.
Gump, B.B., Wu, Q., Dumas, A.K. and Kannan, K. (2011). Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ. Sci. Technol., 45(19): 8151-8159.
Guruge, K.S., Hikono, H., Shimada, N., Murakami, K., Hasegawa, J., Yeung, L.W., et al. (2009). Effect of perfluorooctane sulfonate (PFOS) on influenza A virus-induced mortality in female B6C3F1 mice. J. Toxicol. Sci., 34(6): 687-691.
Gützkow, K.B., Haug, L.S., Thomsen, C., Sabaredzovic, A., Becher, G. and Brunborg, G. (2012). Placental transfer of perfluorinated compounds is selective-a Norwegian Mother and Child sub-cohort study. Int. J. Hyg. Environ. Health, 215(2): 216-219.
Haber, L.T., Dourson, M.L. and Mohapatra A. (2013). Development of chemical-specific adjustment factors for long-lived chemicals: PFOS as a model chemical. Poster presented at Society for Risk Analysis Annual Meeting, Baltimore, MD, December 8-11.
Halldorsson, T.I., Rytter, D., Haug, L.S., Bech, B.H., Danielsen, I., Becher, G. et al. (2012). Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ. Health Perspect., 120(5): 668-673.
Hamm, M.P., Cherry, N.M., Chan, E., Martin, J.W. and Burstyn, I. (2010). Maternal exposure to perfluorinated acids and fetal growth. J. Expo. Sci. Environ. Epidemiol., 20(7): 589-597.
Hansen, K.J., Johnson, H.O., Eldridge, J.S., Butenhoff, J.L. and Dick, L.A. (2002). Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol.., 36(8): 1681-1685.
Hansen, M.C., Børresen, M.H., Schlabach, M. and Cornelissen, G. (2010). Sorption of perfluorinated compounds from contaminated water to activated carbon. J. Soils Sediments, 10: 179-185.
Harada, K., Saito, N., Sasaki, K., Inoue, K., Koizumi, A. (2003). Perfluoroocatne sulfonate contamination of drinking water in the Tama river, Japan: Estimated effects on resident serum levels. Environ. Contam. Toxicol. 71:31-36.
Harada, K., Saito, N., Inoue, K., Yoshinaga, T., Watanabe, T., Sasaki, S. et al. (2004). The influence of time, sex and geographic factors on levels of perfluorooctane sulfonate and perfluorooctanoate in human serum over the last 25 years. J. Occup. Health, 46(2): 141-147.
Harada, K., Inoue, K., Morikawa, A., Yoshinaga, T. and Saito, N. (2005). Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Env. Res, 99: 653-261.
Harada, K.H., Hashida, S., Kaneko, T., Takenaka, K., Minata, M., Inoue, K. et al. (2007). Biliary excretion and cerebrospinal fluid partition of perfluorooctanoate and perfluorooctane sulfonate in humans. Environ. Toxicol. Pharmacol, 24(2): 134-139.
Hatfield, T.L. (1999). Study of the stability of MeFOSEA in aqueous buffers using gas chomatography with atomic emission detection. AR226-0380. [As cited in Martin et al. (2010)].
Hazleton Laboratories America Inc. (1987). Primary eye irritation study in rabbits - method, summary, raw data appendix. # 70100355, sample T-4016. [As cited in Health Canada (2006)].
Hazleton Wisconsin Inc. (1994). Final report: Primary eye irritation/corrosion study of PFOS (T-5898) in rabbits (OECD Guidelines). # 40200470. [As cited in Health Canada (2006)].
Health Canada. (1994). Human health risk assessment for priority substances. Minister of Supply and Services Canada, Ottawa, Ontario.
Health Canada (2006). State of the science report for a screening health assessment. perfluorooctane sulfonate (PFOS) its salts and its precursors that contain the C8HF17SO2 or C8HF17SO3 moiety. Available at: www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/pfos-spfo/index-eng.php.
Health Canada. (2010). Report on human biomonitoring of environmental chemicals in Canada: results of the Canadian Health Measures Survey Cycle 1 (2007-2009). Her Majesty the Queen in Right of Canada, represented by the Minister of Health, Ottawa, Ontario.
Health Canada. (2013a). National Survey query PFOS-PFOA 2009 and 2010. Excel spreadsheet. Health Canada, Ottawa, Ontario.
Health Canada. (2013b). Second report on human biomonitoring of environmental chemicals in Canada: results of the Canadian Health Measures Survey Cycle 2 (2009-2011). Her Majesty the Queen in Right of Canada, represented by the Minister of Health, Ottawa, Ontario.
Health Canada. (2013c). Seacat reanalysis-statistical analysis of cynomolgus monkey data. Internal report. Health Canada, Ottawa, Ontario.
Herzke, D., Olsson, E. and Posner, S. (2012). Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in consumer products in Norway-a pilot study. Chemosphere, 88(8): 980-987.
Higgins, C.P. and Luthy, R.G. (2006). Sorption of perfluorinated surfactants on sediments. Environ. Sci. Technol. 40: 7251-7256.
Hoffman, K., Webster, T.F., Weisskopf, M.G., Weinberg, J. and Vieira, V.M. (2010). Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12-15 years of age. Environ. Health Perspect., 118(12): 1762-1767.
Hölzer, J., Midasch, O., Rauchfuss, K., Kraft, M., Reupert, R., Angerer, J., Kleeschulte, P., Marschall, N. and Wilhelm, M. (2008). Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ. Health Perspect.., 116(5): 651-657.
Hori, H., Hayakawa, E., Einaga, H., Kutsuna, S., Koike, K., Ibusuki, T., Kiatagawa, H. and Arakawa, R. (2004a). Decomposition of environmentally persistent pefluorooctanoic acid in water by photochemical approaches. Environ. Sci. Technol., 38: 6118-6124.
Hori, H., Hayakawa, E., Yamashita, N., Taniyasu, S., Nacata, F. and Kobayashi, Y. (2004b). High performance liquid chromatography with conductimetric detection of perfluorocarboxilic acids and perfluorosulfonates. Chemosphere 57:273-282.
Hori, H., Yamamoto, A., Hayakawa, E., Einaga, H., Taniyasu, S., Yamashita, N. and Kutsuna, S. (2005). Efficient decomposition of environmentally persistent pefluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol., 39: 2383-2388.
Hori, H., Yamamoto, A., Koike, K., Kutsuna, S., Osaka, I. and Arakawa, R. (2007). Photochemical decomposition of environmentally persistent short-chain perfluorocarboxylic acids in water mediated by iron(II)/(III) redox reactions. Chemosphere, 68: 572-578.
Hundley, S.G., Sarrif, A.M. and Kennedy, G.L. (2006). Absorption, distribution, and excretion of ammonium perfluorooctanoate (APFO) after oral administration to various species. Drug Chem. Toxicol., 29(2): 137-145.
Ingelido, A.M., Marra, V., Abballe, A., Valentini, S., Iacovella, N., Barbieri, P., Porpora, M.G., di Domenico, A. and De Felip, E. (2010). Perfluorooctanesulfonate and perfluorooctanoic acid exposures of the Italian general population. Chemosphere, 80(10): 1125-1130.
Inoue, K., Okada, F., Ito, R., Kato, S., Sasaki, S., Nakajima, S. et al. (2004). Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ. Health Perspect, 112(11): 1204-1207. [As cited in EFSA (2008)].
ISO (2009). ISO 25101 Water quality - Determination of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) - Method for unfiltered water samples using solid phase extraction and liquid chromatography with mass spectrometry. International Standardization Organization.
IPCS (1994). Assessing human health risks of chemicals: derivation of guidance values for health-based exposure limits. International Programme on Chemical Safety, Environmental Health Criteria 170, World Health Organization, Geneva, Switzerland.
IPCS (2005). Chemical-specific adjustment factors for interspecies differences and human variability: guidance document for use of data in dose/concentration-response assessment. International Programme on Chemical Safety, Harmonization Project Document No. 2. World Health Organization, Geneva, Switzerland.
IPCS (2012). Guidance for immunotoxicity risk assessment for chemicals. Harmonization Project Document No. 10. World Health Organization, Geneva, Switzerland.
Jacquet, N., Maire, M.A., Landkocz, Y. and Vasseur, P. (2012). Carcinogenic potency of perfluorooctane sulfonate (PFOS) on Syrian hamster embryo (SHE) cells. Arch. Toxicol., 86(2): 305-314.
Jahnke, A. and Berger, U. (2009). Trace analysis of per- and polyfluorinated alkyl substances in various matrices-How do current method perform? J. Chromatogr. A 1216:410-421.
Japanese Industrial Standard (2011). JIS K 0450-70-10, Testing methods for perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) in industrial water and wastewater.
Joensen, U.N., Bossi, R., Leffers, H., Jensen, A.A., Skakkebaek, N.E. and Jørgensen, N. (2009). Do perfluoroalkyl compounds impair human semen quality? Environ. Health Perspect., 117(6): 923-927.
Johansson, N., Fredriksson, A. and Eriksson, P. (2008). Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology, 29(1): 160-169.
Johansson, N., Eriksson, P. and Viberg, H. (2009). Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol. Sci., 108(2): 412-418.
Johnson, J. D. and Ober, R. E. (1979). Absorption of FC-95-14C in rats after a single oral dose. 3M. Submitted to the U.S. Environmental Protection Agency's Administrative Record. AR226-0007. [As cited in ATSDR (2009), EFSA (2008)].
Johnson, J.D. and Ober, R.E. (1999). Absorption of FC-143-14C in rats after a single oral dose. In: Exploratory 28-day oral toxicity study with telomer alcohol, telomer acrylate, PFHS, and PFOS (POS control) by daily gavage in the rat, w/CVR LTR DTD, 051500 (Sanitized) 3M. Submitted to the U.S. Environmental Protection Agency under TSCA Section FYI. OTS05001378S. [As cited in ATSDR (2009)].
Kadar, H., Veyrand, B., Barbarossa, A., Pagliuca, G., Legrand, A., Bosher, C. et al. (2011). Development of an analytical strategy based on liquid chromatography-high resolution mass spectrometry for measuring perfluorinated compounds in human breast milk: application to the generation of preliminary data regarding perinatal exposure in France. Chemosphere, 85(3): 473-480.
Kannan, K., Corsolini, S., Falandysz, J., Fillmann, G., Kumar, K.S., Loganathan, B.G. et al. (2004). Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol., 38(17):4489-4495. [As cited in Health Canada (2006)].
Karbe, E. and Kerlin, R.L. (2002). Cystic degeneration/spongiosis hepatis in rats. Toxicol. Pathol., 30(2): 216-227.
Kärrman, A., Domingo, J.L., Llebaria, X., Nadal, M., Bigas, E., van Bavel, B. and Lindström G. (2010). Biomonitoring perfluorinated compounds in Catalonia, Spain: concentrations and trends in human liver and milk samples. Environ. Sci. Pollut. Res. Int., 17(3): 750-758.
Kato, K., Wong, L.Y., Jia, L.T., Kuklenyik, Z. and Calafat, A.M. (2011). Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ. Sci. Technol., 45(19): 8037-8045.
Kawamoto, K., Oashi, T., Oami, K., Liu, W., Jin, Y., Saito, N. et al. (2010). Perfluorooctanoic acid (PFOA) but not perfluorooctane sulfonate (PFOS) showed DNA damage in comet assay on Paramecium caudatum. J. Toxicol. Sci., 35(6): 835-841.
Kawamoto, K., Sato, I., Tsuda, S., Yoshida, M., Yaegashi, K., Saito, N., et al. (2011). Ultrasonic-induced tonic convulsion in rats after subchronic exposure to perfluorooctane sulfonate (PFOS). J. Toxicol. Sci., 36(1): 55-62.
Keil, D.E., Mehlmann, T., Butterworth, L. and Peden-Adams, M.M. (2008). Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol. Sci., 103(1): 77-85.
Kemper, R.A. (2003). Perfluorooctanoic acid: Toxicokinetics in the rat. Association of Plastics Manufactures of Europe. Project ID: DuPont 7473. U.S. EPA public docket, administrative record. AR226-1499.
Kemper, R.A. and Nabb, D.L. (2005). In vitro studies in microsomes from rat and human liver, kidney, and intestine suggest that perfluorooctanoic acid is not a substrate for microsomal UDPglucuronosyltransferases. Drug Chem. Toxicol., 28(3): 281-287.
Kim, S., Choi, K., Ji, K., Seo, J., Kho, Y., Park, J., Hwang, I., Jeon, J., Yang, H. and Giesy, J.P. (2011a). Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol., 45(17): 7465-7472.
Kim, H.S., Jun Kwack, S., Sik Han, E., Seok Kang, T., Hee Kim, S. and Young Han, S. (2011b). Induction of apoptosis and CYP4A1 expression in Sprague-Dawley rats exposed to low doses of perfluorooctane sulfonate. J. Toxicol. Sci., 36(2): 201-210.
Knox, S.S., Jackson, T., Javins, B., Frisbee, S.J., Shankar, A. and Ducatman, A.M. (2011a). Implications of early menopause in women exposed to perfluorocarbons. J. Clin. Endocrinol. Metab., 96(6): 1747-1753.
Knox, S.S., Jackson, T., Frisbee, S.J., Javins, B. and Ducatman, A.M. (2011b). Perfluorocarbon exposure, gender and thyroid function in the C8 Health Project. J. Toxicol. Sci., 36(4): 403-410.
Koibuchi, N. and Chin, W.W. (2000). Thyroid hormone action and brain development. Trends Endocrinol. Metab., 11(4): 123-128.
Kolstad, C. (2010). GAC treatment for PFCs in Oakdale. Breeze, 143, 14-15.
Krishnan, K. and Carrier, R. (2008). Approaches for evaluating the relevance of multiroute exposures in establishing guideline values for drinking water contaminants. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev., 26(3): 300-316.
Krishnan, K. and Carrier, R. (2013). The use of exposure source allocation factor in the risk assessment of drinking-water contaminants. J. Toxicol. Environ. Health B Crit. Rev., 16(1): 39-51.
Kubwabo, C., Vais, N. and Benoit, F.M. (2004). A pilot study on the determination of perfluorooctanesulfonate and other perfluorinated compounds in blood of Canadians. J. Environ. Monit., 6(6): 540-545.
Kubwabo, C., Stewart, B., Zhu, J. and Marro, L. (2005). Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. J. Environ. Monit., 7(11): 1074-1078. [As cited in EFSA (2008); ATSDR (2009); Environment Canada and Health Canada (2012)].
La Rocca, C., Alessi, E., Bergamasco, B., Caserta, D., Ciardo, F., Fanello, E., Focardi, S., Guerranti, C., Stecca, L., Moscarini, M., Perra, G., Tait, S., Zaghi, C. and Mantovani, A. (2012). Exposure and effective dose biomarkers for perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in infertile subjects: preliminary results of the PREVIENI project. Int. J. Hyg. Environ. Health, 215(2): 206-211.
Lampert, D.J., Frisch, M.A. and Speitel.Jr, G.E. (2007). Removal of perfluorooctanoic acid and perfluorooctanesulfonate from wastewater by ion exchange. Practice Periodical of Hazardous, Toxic, and Radioactive Waste Management. 11: 60-68.
Lange, F.T., Schmidt, C. and Brauch, H-J.(2006). Perfluoroalkyl carboxylates and sulfonates, Rhine Water Works, The Netherlands, Association of River Waterworks - RIWA.
Larsen, B.S. and Kaiser, M.A., 2007. Challenges in perfluorocarboxylic acid measurement. Anal. Chem., 79: 3966-3973.
Lau, C., Thibodeaux, J.R., Hanson, R.G., Rogers, J.M., Grey, B.E., Stanton, M.E., Butenhoff, J.L. and Stevenson, L.A. (2003). Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol. Sci., 74(2):382-392.
Lau, C., Anitole, K., Hodes, C., Lai, D., Pfahles-Hutchens, A. and Seed, J. (2007). Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci., 99: 366-394.
Lefebvre, D.E., Curran, I., Armstrong, C., Coady, L., Parenteau, M., Liston, V., et al. (2008). Immunomodulatory effects of dietary potassium perfluorooctane sulfonate (PFOS) exposure in adult Sprague-Dawley rats. J. Toxicol. Environ. Health A., 71(23): 1516-1525.
Lindstrom, A.B., Strynar, M.J., Delinsky, A.D., Nakayama, S.F., McMillan, L., Libelo, E.L., Neill, M. and Thomas, L. (2011). Application of WWTP biosolids and resulting perfluorinated compound contamination of surface and well water in Decatur, Alabama, USA. Environ. Sci. Technol., 45(19): 8015-8021.
Lipp, P., Sacher, F., Baldauf, G. (2010). Removal of organic micro-pollutants during drinking water treatment by nanofiltration and reverse osmosis. Desalination Water Treat., 13(13): 226-237.
Little Hocking Water Association (2010). GAC filter C-8 sampling result summary. Available at: http://littlehockingwater.org/newsite/?cat=8
Liu, L., Liu, W., Song, J., Yu, H., Jin, Y., Oami, K. et al. (2009a). A comparative study on oxidative damage and distributions of perfluorooctane sulfonate (PFOS) in mice at different postnatal developmental stages. J. Toxicol. Sci., 34(3): 245-254.
Liu, L., Jin, Y.H., Wang, L., Yu, H.Y., Liu, W., Yu, Q.L. et al. (2009b). [Effects of perfluorooctane sulfonate on learning and memory of rat pups]. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese journal of preventive medicine], 43(7): 622-627. Chinese paper. Abstract only.
Liu, X., Liu, W., Jin, Y., Yu, W., Liu, L. and Yu, H. (2010a). Effects of subchronic perfluorooctane sulfonate exposure of rats on calcium-dependent signaling molecules in the brain tissue. Arch. Toxicol., 84(6): 471-479.
Liu, X., Liu, W., Jin, Y., Yu, W., Wang, F. and Liu, L. (2010b). Effect of gestational and lactational exposure to perfluorooctanesulfonate on calcium-dependent signaling molecules gene expression in rats' hippocampus. Arch. Toxicol., 84(1): 71-79.
Liu, J., Li, J., Liu, Y., Chan, H.M., Zhao, Y., Cai, Z. and Wu, Y. (2011). Comparison on gestation and lactation exposure of perfluorinated compounds for newborns. Environ. Int., 37(7): 1206-1212.
Loccisano, A.E., Campbell, J.L., Jr., Andersen, M.E. and Clewell, H.J., 3rd. (2011). Evaluation and prediction of pharmacokinetics of PFOA and PFOS in the monkey and human using a PBPK model. Regul. Toxicol. Pharmacol., 59(1): 157-175.
Loccisano, A.E., Campbell, J.L., Jr., Butenhoff, J.L., Andersen, M.E. and Clewell, H.J., 3rd. (2012a). Comparison and evaluation of pharmacokinetics of PFOA and PFOS in the adult rat using a physiologically based pharmacokinetic model. Reprod. Toxicol., 33(4): 452-467.
Loccisano, A.E., Campbell, J.L., Jr., Butenhoff, J.L., Andersen, M.E. and Clewell, H.J., 3rd. (2012b). Evaluation of placental and lactational pharmacokinetics of PFOA and PFOS in the pregnant, lactating, fetal and neonatal rat using a physiologically based pharmacokinetic model. Reprod. Toxicol., 33(4): 468-90.
Loccisano, A.E., Longnecker, M.P., Campbell, J.L., Jr., Andersen, M.E. and Clewell, H.J., 3rd. (2013). Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J. Toxicol. Environ. Health A, 76(1): 25-57.
Long, Y., Wang, Y., Ji, G., Yan, L., Hu, F. and Gu, A. (2013). Neurotoxicity of perfluorooctane sulfonate to hippocampal cells in adult mice. PLoS One, 8(1): e54176.
Loos.R., Wollgast, J., Huber, T. and Hanke, G. (2007). Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around Lake Maggiore in Northern Italy. Anal. Bioanal. Chem., 387:1469-1478.
Lopez-Espinosa, M.J., Fletcher, T., Armstrong, B., Genser, B., Dhatariya, K., Mondal, D. et al. (2011). Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ. Sci. Technol.., 45(19): 8160-8166.
Lopez-Espinosa, M.J., Mondal, D., Armstrong, B., Bloom, M.S. and Fletcher, T. (2012). Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ. Health Perspect., 120(7): 1036-1041.
Lowen, M., Halldorson, T., Wang, F, and Tomy, Gregg. (2005). Fluorotelomer carboxylic acids and PFOS in rainwater from urban center in Canada. Environ. Sci. Technol. 39: 2944-2951.
Luebker, D.J., Hansen, K.J., Bass, N.M., Butenhoff, J.L. and Seacat, A.M. (2002). Interactions of fluorochemicals with rat liver fatty acid-binding protein. Toxicology, 176(3): 175-185.
Luebker, D.J., Case, M.T., York, R.G., Moore, J.A., Hansen, K.J. and Butenhoff, J.L. (2005a). Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology, 215(1-2): 126-148.
Luebker, D.J., York, R.G., Hansen, K.J., Moore, J.A. and Butenhoff, J.L. (2005b). Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: dose-response, and biochemical and pharamacokinetic parameters. Toxicology, 215(1-2): 149-169.
Maestri, L., Negri, S., Ferrari, M., Ghittori, S., Fabris, F., Danesino, P. and Imbriani, M. (2006). Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun. Mass Spectrom., 20(18):2728-2734.
Maisonet, M., Terrell, M.L., McGeehin, M.A., Christensen, K.Y., Holmes, A., Calafat, A.M. and Marcus, M. (2012). Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ. Health Perspect., 120(10): 1432-1437.
Mak, Y.L., Taniyasu, S., Yeung, L.W.Y., Lu, G.H., Jin, L., Yang, Y.L., Lam, P.K.S., Kannan, K. and Yamashita, N. (2009). Perfluorinated compounds in tap water from China and several other countries. Environ. Sci. Technol. 43(13): 4824-4829.
Martin, J., Kannan, K., Berser, U., deVoogt, P., Field, J., Franklin, J., Giesy, J., Harner, T., Muir, D., Scott, B., Kaiser, M., Jarnberg, U., Jones, K., Mabury, S., Schroeder, H., Simcik, M., Sottani, C., Van Bavel., B., Karrmane, A., Lindstrom, G. and Van Leeuwen, S. (2004). Analytical challenges hamper perfluoroalkyl research. Environ. Sci. Technol., 38(13): 248A-255A.
Martin, M.T., Brennan, R.J., Hu, W., Ayanoglu, E., Lau, C., Ren, H., Wood, C.R., Corton, J.C., Kavlock, R.J. and Dix, D.J. (2007). Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol. Sci., 97(2): 595-613.
Martin, J. W., Asher, B. J., Beesoon, S., Benskin, J. P. and Ross, M. S. (2010). PFOS or PreFOS? Are perfluorooctanesulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctanesulfonate (PFOS) exposure? J. Environ. Monitor., 12(11), 1979-2004.
MDH (2008a). Public 1071 health assessment: perfluorochemical 1072 contamination in Lake Elmo and Oakdale, Washington County, Minnesota. Minnesota Department of Health. Available at: www.health.state.mn.us/divs/eh/wells/waterquality/poudevicefinal.pdf
MDH (2008b). Performance evaluation: Removal of perfluorochemicals (PFC's) with point-of-use (POU) water treatment devices. Final report, prepared by Philip C. Olsen and David J. Paulson, Water Science & Marketing, LLC for the Minnesota Department of Health. May. Available at: www.health.state.mn.us/divs/eh/wells/waterquality/poudevicefinal.pdf
MDH (2008c). Health risk limits for perfluorochemicals. Report to the Minnesota legislature. Final report. Minnesota Department of Health. January.
MDH (2008d). Health risk limits for groundwater 2008 rule revision. Perfluorooctanate sulfonate. Minnesota Department of Health.
Meek, M.E., Palermo, C.M., Bachman, A.N., North, C.M. and Lewis, J.R. (2014). Mode of action human relevance (species concordance) framework: Evolution of the Bradford Hill considerations and comparative analysis of weight of evidence. J. Appl. Toxicol., 34(6): 595-606.
Melzer, D., Rice, N., Depledge, M.H., Henley, W.E. and Galloway, T.S. (2010). Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ. Health Perspect, 118(5): 686-692.
Mendel, A. (1977). Technical report: Analytical methodology on FM 3422. AR226-0364. [As cited in Martin et al. (2010)].
Midasch, O., Drexler, H., Hart, N., Beckmann, M.W. and Angerer, J. (2007). Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int. Arch. Occup. Environ. Health, 80(7): 643-648.
Ministère du Développement durable, de l'Environnement, de la Faune et des Parcs. (2012). Les composés perfluorés dans les cours d'eau et l'eau potable du Québec méridonal. Gouvernement du Québec, Québec, Québec.
Mollenhauer, M.A., Bradshaw, S.G., Fair, P.A., McGuinn, W.D. and Peden-Adams, M.M. (2011). Effects of perfluorooctane sulfonate (PFOS) exposure on markers of inflammation in female B6C3F1 mice. J Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 46(2): 97-108.
Mondal, D., Lopez-Espinosa, M.J., Armstrong, B., Stein, C.R. and Fletcher, T. (2012). Relationships of perfluorooctanoate and perfluorooctane sulfonate serum concentrations between mother-child pairs in a population with perfluorooctanoate exposure from drinking water. Environ. Health Perspect., 120(5): 752-757.
Monroy, R., Morrison, K., Teo, K., Atkinson, S., Kubwabo, C., Stewart, B. and Foster, W.G. (2008). Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ. Res., 108(1): 56-62.
Moody, C.A., Kwan, W.C., Martin, J.W., Muir, D.C.G. and Mabury, S.A. (2001). Determination of perfluorinated surfactants in surface water samples by two independent analytical techniques: liquid chromatography/ tandem mass spectrometry and 19F NMR. Anal. Chem., 73: 2200-2206.
Moody, C.A., Hebert, G.N., Strauss, S.H. and Field, J.A. (2003). Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. J. Environ. Monit., 5(2): 341-345.
Morreale de Escobar, G., Jesús Obregón, M. and Escobar del Rey, F. (2004). Role of thyroid hormone during early brain development. Eur. J. Endocrinol., 151: U25-U37.
Nakayama, S., Strynar, M.., Helfant, L., Egeghy, P., Ye, X. and Lindstrom, A. (2007). Perfluorinated compounds in the Cape Fear Drainage basin in North Carolina. Environ. Sci. Technol. 41:5271-5276.
Needham, L. L., Grandjean, P., Heinzow, B., Jørgensen, P.J., Nielsen, F., Patterson, D. G., Jr., Sjödin, A., Turner, W.E. and Weihe, P. 2011. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol., 45(3): 1121-1126.
Nelson, J.W., Hatch, E.E. and Webster, T.F. (2010). Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect., 118(2): 197-202.
NOTOX (1999). Exploratory 28-day oral toxicity study with telomer alcohol, telomer acrylate, [redacted confidential business information], PFHS and PFOS (positive control) by daily gavage in the rat followed by a 14/28-day recovery period. # 242933. [As cited in Health Canada (2006)].
NSF/ANSI (2014). NSF International/American National Standards Institute Standard 61: Drinking water system components-health effects. NSF International, Ann Arbor, Michigan.
Ochoa-Herrera, V. and Sierra-Alvarez, R. (2008). Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere, 72: 1588-1593.
Oda, Y., Nakayama, S., Harada, K.H. and Koizumi, A. (2007). Negative results ofumu genotoxicity test of fluorotelomer alcohols and perfluorinated alkyl acids. Environ. Health Prev. Med., 12(5): 217-219.
OECD (2002). Co-operation on existing chemicals hazard assessment of perfluorooctane sulfonate (PFOS) and its salts. Environment directorate joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology. Available at: www.oecd.org/chemicalsafety/risk-assessment/2382880.pdf.
Okada, E., Sasaki, S., Saijo, Y., Washino, N., Miyashita, C., Kobayashi, S. et al. (2012). Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants. Environ. Res., 112: 118-125.
Olsen, G.W., Burlew, M.M., Hocking, B.B., Skratt, J.C., Burris, J.M. and Mandel, J.H. (2001). An epidemiologic analysis of episodes of care of 3M Decatur chemical and film plant employees, 1993-1998. Final Report May 18. [As cited in EFSA (2008)].
Olsen, G.W., Hansen, K.J., Stevenson, L.A., Burris, J.M. and Mandel, J.H. (2003a). Human donor liver and serum concentrations of perfluorooctanesulfonate and other perfluorochemicals. Environ. Sci. Technol., 37:888-891.
Olsen, G.W., Butenhoff, J.L. and Mandel, J.N. (2003b). Assessment of lipid, hepatic and thyroid function in relation to an occupational biologic limit value for perfluorooctanoate. 3M Company. Final Report. June 9. U.S. EPA AR226-1351.
Olsen, G.W., Burris, J.M., Ehresman, D.J., Froehlich, J.W., Seacat, A.M., Butenhoff, J.L. et al. (2007). Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect., 115(9): 1298-1305.
Olsen, G., Ehresman, D.J., Buehrer, B.D., Gibson, B.A., Butenhoff, J.L. and Zobel, L.R. (2012). Longitudinal assessment of lipid and hepatic clinical parameters in workers involved with the demolition of perfluoroalkyl manufacturing facilities. J. Occup. Environ. Med., 54(8): 974-983.
Onishchenko, N., Fischer, C., Wan Ibrahim, W.N., Negri, S., Spulber, S., Cottica, D. and Ceccatelli, S. (2011). Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox. Res., 19(3): 452-461.
Ostertag, S.K., Chan, H.M., Moisey, J., Dabeka, R. and Tittlemier, S.A. (2009a). Historic dietary exposure to perfluorooctane sulfonate, perfluorinated carboxylates, and fluorotelomer unsaturated carboxylates from the consumption of store-bought and restaurant foods for the Canadian population. J. Agric. Food Chem., 57(18): 8534-8544.
Ostertag, S.K., Tague, B.A., Humphries, M.M., Tittlemier, S.A. and Chan, H.M. (2009b). Estimated dietary exposure to fluorinated compounds from traditional foods among Inuit in Nunavut, Canada. Chemosphere, 75(9): 1165-1172.
Panchangam, S. C., Yu-Chen Lin, A., Shaik, K.L. and, Lin, C-F.(2009). Decomposition of perfluorocarboxylic acids (PFCAs) by heterogeneous photocatalysis in acidic aqueous medium. Chemosphere 77: 242-248.
Peden-Adams, M.M., Keller, J.M., Eudaly, J.G., Berger, J., Gilkeson, G.S. and Keil, D.E. (2008). Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci., 104(1): 144-154.
Pirali, B., Negri, S., Chytiris, S., Perissi, A., Villani, L., La Manna, L., Cottica, D., Ferrari, M., Imbriani, M., Rotondi, M. and Chiovato, L. (2009). Perfluorooctane sulfonate and perfluorooctanoic acid in surgical thyroid specimens of patients with thyroid diseases. Thyroid, 19(12): 1407-1412.
Pop, V.J., Kuijpens, J.L., van Baar, A.L., Verkerk, G., van Son, M.M., de Vijlder, J.J., Vulsma, T., Wiersinga, W.M., Drexhage, H.A. and Vader, H.L. (1999). Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin. Endocrinol., 50(2): 149-155.
Post, G., Louis, J., Lippincott, L. and Procopio, N. (2013). Occurrence of perfluorinated compounds in raw water from New Jersey public drinking water system. Environ. Sci. Technol. 47:13266-13275.
Prevedouros , K., Cousins, I.T., Buck, R.C. and Korzeniowski, S.H. (2006). Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 40 (1): 32-44.
Qazi, M.R., Bogdanska, J., Butenhoff, J.L., Nelson, B.D., DePierre, J.W. and Abedi-Valugerdi, M. (2009a). High-dose, short-term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion. Toxicology, 262(3): 207-214.
Qazi, M.R., Xia, Z., Bogdanska, J., Chang, S.C., Ehresman, D.J., Butenhoff, J.L., et al. (2009b). The atrophy and changes in the cellular compositions of the thymus and spleen observed in mice subjected to short-term exposure to perfluorooctanesulfonate are high-dose phenomena mediated in part by peroxisome proliferator-activated receptor-alpha (PPARalpha). Toxicology., 260(1-3):68-76.
Qazi, M.R., Abedi, M.R., Nelson, B.D., DePierre, J.W. and Abedi-Valugerdi, M. (2010a). Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int Immunopharmacol., 10(11): 1420-1427.
Qazi, M.R., Nelson, B.D., Depierre, J.W. and Abedi-Valugerdi, M. (2010b). 28-Day dietary exposure of mice to a low total dose (7 mg/kg) of perfluorooctanesulfonate (PFOS) alters neither the cellular compositions of the thymus and spleen nor humoral immune responses: does the route of administration play a pivotal role in PFOS-induced immunotoxicity? Toxicology, 267(1-3): 132-139.
Quinones, O. and Snyder, S. (2009). Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ. Sci. Technol., 43(24): 9089-9095.
Rahman, M.F., Peldszus, S. and Anderson, W.B. (2014). Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Res. 50:318-340.
Raymer, J.H., Michael, L.C., Studabaker, W.B., Olsen, G.W., Sloan, C.S., Wilcosky, T. et al. (2012). Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) and their associations with human semen quality measurements. Reprod. Toxicol., 33(4): 419-427.
Riker Laboratories Inc. (1981). Acute ocular irritation test with T-2997CoC in albino rabbits. # 0882EB0009. [As cited in Health Canada (2006)].
Ritter, L., Totman, C., Krishnan, K., Carrier, R., Vézina, A. and Morisset, V. (2007). Deriving uncertainty factors for threshold chemical contaminants in drinking water. J. Toxicol. Environ. Health B Crit. Rev., 10(7): 527-557.
Rogers, J.M., Ellis-Hutchings, R.G., Grey, B.E., Zucker, R.M., Norwood, J., Jr, Grace, C.E., Gordon, C.J. and Lau, C. (2014). Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol. Sci., 137(2): 436-446.
Roosens, L., D'Hollander, W., Bervoets, L., Reynders, H., Van Campenhout, K., Cornelis, C., Reynders, H., Campenhout, K.V., Voogt, P.D. and Bervoets, L. (2010). Brominated flame retardants and perfluorinated chemicals, two groups of persistent contaminants in Belgian human blood and milk. Environ Pollut., 158(8): 2546-2552.
Rosen, M.B., Schmid, J.R., Corton, J.C., Zehr, R.D., Das, K.P., Abbott, B.D. and Lau, C. (2010). Gene expression profiling in Wild-Type and PPARα-null mice exposed to perfluorooctane sulfonate reveals PPARα-independent effects. PPAR Res. 2010: 1-13.
Rosen, M.B., Das, K.P., Wood, C.R., Wolf, C.J., Abbott, B.D. and Lau, C. (2013). Evaluation of perfluoroalkyl acid activity using primary mouse and human hepatocytes. Toxicology, 308: 129-137.
Rumsby, P., McLaughlin, C. and Hall, T.(2009). Perfluorooctane sulphonate and perfluorooctanoic acid in drinking and environmental waters. Phil. Trans. Royal. Soc. 367:4119-4136.
Rusch, G. (1979). An acute inhalation study of T-2305 CoC in the rat. Bio/dynamics, Inc., Study No. 78-7184, May 3, 1979. U.S. Environmental Protection Agency Administrative Record 226-0417. [As cited in OECD (2002); EFSA (2008)].
Sanexen Environmental Services Inc. (2013). Review of toxicological information on perfluorooctane sulfonate (PFOS) to be used in the technical document for drinking water guidelines. Final contract report to Health Canada.
Sato, I., Kawamoto, K., Nishikawa, Y., Tsuda, S., Yoshida, M., Yaegashi, K. et al. (2009). Neurotoxicity of perfluorooctane sulfonate (PFOS) in rats and mice after single oral exposure. J. Toxicol. Sci., 34(5): 569-574.
SCC (2015). Accredited certification bodies. Standards Council of Canada. Available at: www.scc.ca/en/accreditation/product-process-and-service-certification/directory-of-accredited-clients
Schroeder, A.C. and Privalsky, M.L. (2014). Thyroid hormones, T3 and T4, in the brain. Front. Endocrin., 5(40), 1-6.
Schultz, M., Barofsky, D. and Field, J. (2006). Quantitative determination of fluorinated alkyl substances by large-volume-injection liquid chromatography tandem mass spectrometryscharacterization of municipal wastewaters. Environ. Sci. Technol. 40:289-295.
Seacat, A.M., Thomford, P.J., Hansen, K.J., Clemen, L.A., Eldridge, S.R., Elcombe, C.R. and Butenhoff, J.L. (2003). Sub-chronic dietary toxicity of potassium perfluorooctane sulfonate in rats. Toxicology, 183(1-3): 117-131. Erratum in: Toxicology, 192(2-3):263-264.
Seacat, A.M., Thomford, P.J., Hansen, K.J., Olsen, G.W., Case, M.T. and Butenhoff, J.L. (2002). Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in Cynomolgus monkeys. Toxicol. Sci., 68(1): 249-264.
Senevirathna, S.T.M.L.D, Tanaka, S., Fujii, S., Kunacheva, C., Harada, H., Shivakoti, B.R. and Okamoto, R. (2010). A comparative study of adsorption of perfluorooctanesulfonate (PFOS) onto granular activated carbon, ion-exchange polymers and non-ion-exchange polymers. Chemosphere 80: 647-651.
Senevirathna, S.T.M.L.D, Tanaka, S., Fujii, S., Kunacheva, C., Harada, H., Shivacoti, B., Dinh, H. and Ariadas, T. (2011). Adsorption of four perfluorinated acids on non-ion exchange polymers sorbents. Water Sci. Technol., 63(10): 2106.
Shankar, A., Xiao, J. and Ducatman, A. (2011). Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am. J. Epidemiol., 174(8): 893-900.
Shivakoti, B.R., Fujii, S., Nozoe, M., Tanaka, S. and Kunacheva, C. (2010). Perfluorinated chemicals (PFCs) in water purification plants (WPPs) with advanced treatment processes. Wa. Sci. Technol, 10(1): 87-95.
Shoeib, M., Harner, T., G, M.W. and Lee, S.C. (2011). Indoor sources of poly- and perfluorinated compounds (PFCS) in Vancouver, Canada: implications for human exposure. Environ. Sci. Technol., 45(19): 7999-8005.
Shoemaker, J., Boutin, B. and Grimmett, P. (2009). Development of a U.S. EPA drinking water method for the analysis of selected perfluoroalkyl acids by solid-phase extraction and LC-MS-MS. J. Chromatogr. Sci., 47(1):3-11.
So, M.K., Yamashita, N., Taniyasu, S., Jiang, Q., Giesy, J.P., Chen, K. and Lam, P.K. (2006). Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ. Sci. Technol., 40(9): 2924-2929. Comment in: Environ. Sci. Technol., 40(9): 2866-2867.
Sonia, T.A. and Sharma, C.P. (2011). Chitosan and its derivatives for drug delivery perspective. Adv. Polym. Sci., 243: 23-54.
Sonthithai, P., Suriyo, T., Thiantanawat, A., Watcharasit, P., Ruchirawat, M. and Satayavivad, J. (2015). Perfluorinated chemicals, PFOS and PFOA, enhance the estrogenic effects of 17β-estradiol in T47D human breast cancer cells. J. Appl. Toxicol. Accepted for publication. DOI: 10.1002/jat.3210.
Steenland, K., Tinker, S., Frisbee, S., Ducatman, A. and Vaccarino, V. (2009). Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J. Epidemiol., 170(10): 1268-1278.
Steenland, K., Tinker, S., Shankar, A. and Ducatman, A. (2010). Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ. Health Perspect., 118(2): 229-233.
Stein, C.R. and Savitz, D.A. (2011). Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5-18 years of age. Environ. Health Perspect., 119(10): 1466-1471.
Stein, C.R., Savitz, D.A. and Dougan, M. (2009). Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am. J. Epidemiol., 170(7): 837-846. Comment in: Am. J. Epidemiol., 171(1):131-132; author reply 132-133.
Steinle-Darling, E. and Reinhard, M. (2008). Nanofiltration for trace organic contaminant removal: structure, solution, and membrane fouling effects on the rejection of perfluorochemicals. Environ. Sci. Technol., 42, 5292-5297.
Summit Toxicology (2015). Interspecies extrapolation for perfluorooctyl sulfonate (PFOS) and perfluorooctanoic acid (PFOA). Summit Toxicology, L.L.P. Report submitted to Health Canada.
Sun., H., LI, F., Zhang, T., Zhang, X., He., N., Song, Q., Zhao, L., Sun, L. and Sun, T. (2011). Perfluorinated compounds in surface waters and WWTPs in Shenyang, China: Mass flows and source analysis. Water Res., 45:4483-4490.
Sundström, M., Ehresman, D.J., Bignert, A., Butenhoff, J.L., Olsen, G.W., Chang, S.C. and Bergman, A. (2011). A temporal trend study (1972-2008) of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in pooled human milk samples from Stockholm, Sweden. Environ. Int., 37(1):178-183.
Szostek, B., Prickett, K.B. and Buck, R.C.(2006). Determination of fluorotelomer alcohol by liquid chromatography/tandem mass spectrometry in water. Rapid Commun. Mass Spectrom., 20: 2837.
Takacs, M.L. and Abbott, B.D. (2007). Activation of mouse and human peroxisome proliferator-activated receptors (α, β/δ, γ) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci., 95(1): 108-117.
Takagi, S., Adachi, F., Miyano, K., Koizumi, Y., Tanaka, H., Watanabe, I., Tanabe, S. and Kannan, K. (2011). Fate of perfluorooctanesulfonate and perfluorooctanoate in drinking water treatment processes. Water Res., 45( 13): 3925-3932.
Takagi,S., Adachi, F., Miyano, K., Koizumi Ya, Tanaka, H., Mimura, M., Watanabe, I., Tanabe, S. and Kannan, K. (2008). Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan. Chemosphere, 72: 1409-1412.
Tan, Y.M., Clewell, H.J., 3rd and Andersen, M.E. (2008). Time dependencies in perfluorooctylacids disposition in rat and monkeys: a kinetic analysis. Toxicol. Lett., 177(1): 38-47.
Tang, C.Y., Fu, Q.S., Robertson, A.P., Criddle, C.S. and Leckie, J.O. (2006). Use of reverse osmosis membranes to remove perfluorooctanesulfonate (PFOS) from semiconductor wastewater. Environ. Sci. Technol., 40: 7343-7349.
Tang, C.Y., Shiangfu, Q., Criddle, C.S. and Leckie, J.O. (2007). Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctanesulfonate containing wastewater. Environ. Sci. Technol., 41: 2008-2014.
Tang, C., Kwon, Y-N. and Leckie, J. (2009a). Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination, 242: 149-167.
Tang, C., Kwon, Y-N. and Leckie, J. (2009b). Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes II. Membrane physiochemical properties and their dependence on polyamide and coating layers. Desalination, 242: 168-182.
Tang, H., Xiang, Q., Lei, M., Yan, J., Zhu, L. and Zou, J. (2012). Efficient degradation of perfluorooctanoic acid by UV-Fenton process. Chem. Eng. J., 184: 156-162.
Taniyasu, S., Kannan, K., So, M.K., Gulkowska, A., Sinclair, E., Okazawa, T. and Yamashita, N. (2005). Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J. Chromatogr. A, 1093: 89-97.
Taniyasu, S., Kannan, K., Wu, Q., Kwok, K.Y., Yeung, L.W.Y., Lam, P.K.S., Chittin, B., Kida, T., Takasagu, T., Tsuchiya, Y. and Yamashita, N. (2013). Inter-laboratory trials for analysis of perfluooroocatanesulfonate and perfluorooctanoate in water samples: Performance and recommendations. Anal. Chim. Acta, 770: 111-120.
Tao, L., Kannan, K., Wong, C.M., Arcaro, K.F. and Butenhoff, J.L. (2008). Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ. Sci. Technol., 42(8): 3096-3101.
Thibodeaux, J.R., Hanson, R.G., Rogers, J.M., Grey, B.E., Barbee, B.D., Richards, J.H., Butenhoff ,J.L., Stevenson, L.A. and Lau, C. (2003). Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol. Sci., 74(2): 369-381.
Thomford, P. (2002). Final report: 104 week dietary chronic toxicity and carcinogenicity study with perfluorooctane sulfonic acid potassium salt (PFOS: T-6295) in rats. As cited in EFSA (2008), Health Canada (2012).
Thompson, J., Lorber, M., Toms, L.M., Kato, K., Calafat, A.M. and Mueller, J.F. (2010). Use of simple pharmacokinetic modeling to characterize exposure of Australians to perfluorooctanoic acid and perfluorooctane sulfonic acid. Environ Int., 36(4): 390-397. Erratum in: Environ Int. 36(6): 647-648.
Thompson, J., Eaglesham, G., Reungoat, J., Poussade, Y., Bartkow, M., Lawrence, M. and Mueller, J. F. (2011). Removal of PFOS, PFOA and other perfluoroalkyl acids at water reclamation plants in South East Queensland Australia. Chemosphere 82(1), 9-17.
Thomsen, C., Haug, L.S., Stigum, H., Frøshaug, M., Broadwell, S.L. and Becher, G. (2010). Changes in concentrations of perfluorinated compounds, polybrominated diphenyl ethers, and polychlorinated biphenyls in Norwegian breast-milk during twelve months of lactation. Environ. Sci. Technol.., 44(24): 9550-9556. Erratum in: Environ. Sci. Technol.., 45(7):3192.
Tittlemier, S., Ryan, J.J. and VanOostdam, J. (2004). Presence of anionic perfluorinated organic compounds in serum collected from Northern Canadian populations. Organohalogen Compd 66: 4009-4014. [As cited in Health Canada (2006, 2012)].
Tittlemier, S.A., Pepper, K., Seymour, C., Moisey, J., Bronson, R., Cao, X.L. et al. (2007). Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem., 55(8): 3203-3210.
Toft, G., Jönsson, B.A., Lindh, C.H., Giwercman, A., Spano, M., Heederik, D. et al. (2012). Exposure to perfluorinated compounds and human semen quality in Arctic and European populations. Hum. Reprod., 27(8): 2532-2540.
Tsai, Y., Yu-Chen Lin, A., Weng, Y. and Li, K. (2010). Treatment of perfluorinated chemicals by electro-microfiltration. Environ. Sci. Technol., 44(20): 7914-7920.
Tucker, D.K., Macon, M.B., Strynar, M.J., Dagnino, S., Andersen, E. and Fenton, S.E. (2015). The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod. Toxicol., 54: 26-36.
Turgeon O'Brien, H., Blanchet, R., Gagné, D., Lauzière, J., Vézina, C., Vaissière, E., Ayotte, P. and Déry, S. (2012). Exposure to toxic metals and persistent organic pollutants in Inuit children attending childcare centers in Nunavik, Canada. Environ. Sci. Technol., 46(8): 4614-23. Erratum in: Environ. Sci. Technol., 46(14): 7926.
UK COT (2006). COT statement on the tolerable daily intake for perfluorooctanoic acid. UK Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. Available at: www.food.gov.uk/multimedia/pdfs/cotstatementpfoa200610.pdf
UK HPA (2007). Maximum acceptable concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in drinking water. Available at: www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947397222
UK HPA (2009). HPA Compendium of chemical hazards. PFOS + PFOA. Version 1.
U.S.EPA (2005). Draft risk assessment of the potential human health effects associated with exposure to perfluorooctanoic acid and its salts. Office of Pollution Prevention and Toxics Risk Assessment Division. Available at: www.epa.gov/oppt/pfoa/pubs/pfoarisk.html
U.S. EPA (2009a). EPA Method 537, Determination of selected perfluorinated alkyl acids in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS). Version 1.1. September 2009. EPA/600/R-08/092.
U.S. EPA (2009b). Provisional health advisories for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). 1-5. US EPA Office of Water.
U.S. EPA (2009c). The toxicity of perfluoroactanoic acid (PFOA) and of percfluorooctanate sulfonate (PFOS). Memorandum. Office of Solid Waste and Emergency Response.
U.S. EPA (2012a). Benchmark dose technical guidance. Report No. EPA/100/R-12/001. Risk Assessment Forum, U.S. Environmental Protection Agency, Washington, DC.
U.S. EPA (2012b). UCMR3 laboratory approval requirements and information document. Version 2. Technical support center. Standards and Risk Management Division. Office of Groundwater and Drinking Water. U.S. Environmental Protection Agency, Cincinnati, Ohio 45268.
U.S. EPA (2015). Benchmark dose software (BMDS) Version 2.6.0.86 [Build: 2/4/2015]. National Center for Environmental Assessment. U.S. Environmental Protection Agency, Washington, DC. Available at: http://bmds.epa.gov.
van Leeuwen, S. P. and de Boer, J. (2007). Extraction and clean-up strategies for the analysis of poly-and perfluoroalkyl substances in environmental and human matrices. J. Chromatogr. A, 1153(1-2):172-185.
van Leeuwen, S.P., Karrman, A., van Bevel, B., de Boer, J. and Lindstrom, G. (2006). Struggle of quality in determination of perfluorinated contaminants in environmental and human samples. Environ. Sci. Technol. 40:7854-7860.
van Leeuwen, S.P.J., Stewart, C.P., van der Veen, I. and de Boer, J., (2009). Significant improvements in the analysis of perfluorinated compounds in water and fish: Results from an interlaboratory method evaluation study. J. Chromatogr. A, 1216: 401-409.
Vanden Heuvel, J.P., Thompson, J.T., Frame, S.R. and Gillies, P.J. (2006). Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol. Sci., 92(2): 476-489.
Vecitis, C.D., Park, H., Cheng, J., Mader, B.T. and Hoffmann, M.R. (2009). Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA). Front. Environ. Sci. Engin. China 2009, 3(2): 129-151.
Vested, A., Ramlau-Hansen, C.H., Olsen, S.F., Bonde, J.P., Kristensen, S.L., Halldorsson, T.I. et al. (2013). Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ. Health Perspect., 121(4): 453-458.
Vestergaard, S., Nielsen, F., Andersson, A.M., Hjøllund, N.H., Grandjean, P., Andersen, H.R. et al. (2012). Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum. Reprod., 27(3): 873-880.
Villagrasa, M., deAlda, M.L. and Barcelo, D. (2006). Environmental analysis of fluorinated alkyl substances by liquid chromatography-(tandem) mass spectrometry: a review. Anal. Bioanal. Chem., 386: 953-972.
Villaverde-de-Saa, E., Fernandez-Lopez, M., Rodil, R., Quintana, J., Racamonde, I. and Cela, R. (2015). Solid phase extraction of perfluoroalkylated compounds from sea water. J. Sep. Sci. 38:1942-1950.
von Ehrenstein, O.S., Fenton, S.E., Kato, K., Kuklenyik, Z., Calafat, A.M. and Hines, E.P. (2009). Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod. Toxicol., 27(3-4):239-245.
Wambaugh, J.F., Setzer, R.W., Pitruzzello, A.M., Liu, J., Reif, D.M., Kleinstreuer, N.C., Wang, N.C., Sipes, N., Martin, M., Das, K., DeWitt, J.C., Strynar, M., Judson, R., Houck, K.A. and Lau C. (2013). Dosimetric anchoring of in vivo and in vitro studies for perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci., 136(2): 308-327.
Wan, H.T., Zhao, Y.G., Wong, M.H., Lee, K.F., Yeung, W.S., Giesy, J.P. et al. (2011). Testicular signaling is the potential target of perfluorooctanesulfonate-mediated subfertility in male mice. Biol. Reprod., 84(5): 1016-1023.
Wang, F., Liu, W., Jin, Y., Dai, J., Yu, W., Liu, X. et al. (2010). Transcriptional effects of prenatal and neonatal exposure to PFOS in developing rat brain. Environ. Sci. Technol., 44(5): 1847-1853.
Wang, F., Liu, W., Jin, Y., Dai, J., Zhao, H., Xie, Q. et al. (2011a). Interaction of PFOS and BDE-47 co-exposure on thyroid hormone levels and TH-related gene and protein expression in developing rat brains. Toxicol. Sci., 121(2): 279-291.
Wang, F., Liu, W., Ma, J., Yu, M., Jin, Y. and Dai, J. (2012). Prenatal and neonatal exposure to perfluorooctane sulfonic acid results in changes in miRNA expression profiles and synapse associated proteins in developing rat brains. Environ. Sci. Technol., 46(12): 6822-6829.
Wang, Y., Liu, W., Zhang, Q., Zhao, H. and Quan, X. (2015). Effects of developmental perfluorooctane sulfonate exposure on spatial learning and memory ability of rats and mechanism associated with synaptic plasticity. Food Chem. Toxicol., 76: 70-76.
Wang, Y., Wang, L., Liang, Y., Qiu, W., Zhang, J., Zhou, Q., et al. (2011b). Modulation of dietary fat on the toxicological effects in thymus and spleen in BALB/c mice exposed to perfluorooctane sulfonate. Toxicol. Lett., 204(2-3): 174-182.
Warf Institute Inc. (1975). Dermal and ocular irritation of PFOS (T-1166) in rabbits. # 5011023. [As cited in Health Canada (2006)].
Washino, N., Saijo, Y., Sasaki, S., Kato, S., Ban, S., Konishi, K. et al. (2009). Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Health Perspect., 1117(4): 660-667.
Weiss, J.M., Andersson, P.L., Lamoree, M.H., Leonards, P.E., van Leeuwen, S.P. and Hamers, T. (2009). Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol. Sci., 109(2): 206-216.
Weiß, O., Wiesmüller, G.A., Bunte, A., Göen, T., Schmidt, C.K., Wilhelm, M. et al. (2012). Perfluorinated compounds in the vicinity of a fire training area-human biomonitoring among 10 persons drinking water from contaminated private wells in Cologne, Germany. Int. J. Hyg. Environ. Health, 215(2): 212-215.
Weremiuk, A. M., Gerstmann, S. and Frank, H. (2006). Quantitative determination of perfluorinated surfactants in water by LC-MS/MS. J. Sep. Sci., 29:2251-2255.
Wetzel, L.T. (1983). Rat teratology study, T-3351, final report. Hazelton Laboratories America, Inc. Project Number: 154-160, December 19, 1983. US EPA AR-226 226-0014. (also cited as Hazleton Laboratories America, Inc.). [As cited in OECD (2002); EFSA (2008): Health Canada (2012)].
Whitworth, K.W., Haug, L.S., Baird, D.D., Becher, G., Hoppin, J.A., Skjaerven, R. et al. (2012). Perfluorinated compounds and subfecundity in pregnant women. Epidemiology, 23(2): 257-63. Comment in: Epidemiology. 23(2): 264-266.
Wilhelm, M., Kraft, M., Rauchfuss, K. and Holzer, J. (2008). Assessment and management of the first German case of a contamination with perfluorinated compounds (PFC) in the region Sauerland, North Rhine Westphalia. J. Toxicol. Environ. Health A,71(11-12): 725-733.
Williams, G.R. (2008). Neurodevelopmental and neurophysiological actions of thyroid hormone. J. Neuroendocrinol., 20: 784-794.
Wolf, S. and Reagen, W. (2011). Method for the determination of perfluotrinated compounds (PFCs) in water by solid-phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS). Anal. Methods, 3:1485-1493.
Xia, W., Wan, Y., Li, Y.Y., Zeng, H., Lv, Z., Li, G. et al. (2011). PFOS prenatal exposure induce mitochondrial injury and gene expression change in hearts of weaned SD rats. Toxicology, 282(1-2): 23-29.
Xiao, F., Davidsavor, K.J., Park, S., Nakayama, M. and Phillips, B.R. (2012). Batch and column study: sorption of perfluorinated surfactants from water and co-solvent systems by Amberlite XAD resins. J. Colloid Interface Sci., 368: 505-511.
Xiao, F., Simcik, M.F. and Gulliver, J.S. (2013). Mechanisms for removal of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) from drinking water by conventional and enhanced coagulation. Water Res., 47: 49-56.
Yahia, D., Tsukuba, C., Yoshida, M., Sato, I. and Tsuda, S. (2008). Neonatal death of mice treated with perfluorooctane sulfonate. J. Toxicol. Sci., 33(2): 219-226.
Yamashita, N., Kannan, K., Taniyasu, S., Horii, Y., Okazawa, T., Petrick, C. and Gamo, T. (2004). Analysis of perflulorinated acids at parts per quadrillion levels in seawater using liquid chromatograph tandem mass spectrometry. Environ. Sci. Technol. 38: 5522-5528.
Ye, L., Zhao, B., Yuan, K., Chu, Y., Li, C., Zhao, C. et al. (2012). Gene expression profiling in fetal rat lung during gestational perfluorooctane sulfonate exposure. Toxicol. Lett., 209(3): 270-276.
Yu, Q., Deng, S. and Yu, G. (2008). Selective removal of perfluorooctanesulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res., .42(12): 3089-3097.
Yu, W.G., Liu, W. and Jin, Y.H. (2009a). Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ. Toxicol. Chem., 28(5): 990-996.
Yu, W.G., Liu, W., Jin, Y.H., Liu, X.H., Wang, F.Q., Liu, L. et al. (2009b). Prenatal and postnatal impact of perfluorooctane sulfonate (PFOS) on rat development: a cross-foster study on chemical burden and thyroid hormone system. Environ. Sci. Technol., 43(21): 8416-8422.
Yu, W.G., Liu, W., Liu, L. and Jin, Y.H. (2011). Perfluorooctane sulfonate increased hepatic expression of OAPT2 and MRP2 in rats. Arch. Toxicol., 85(6): 613-521.
Yu, Q., Zhang, R., Deng, S., Huanga, J. and Yu, G. (2009). Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res., 43(4): 1150-1158.
Zainuddin, K., Zakaria, M. P., Al-Odaini, N., Bakhtiari, A.R and Latif, P.A.,(2012). Perfluorooctanoic acid (PFOA) and perfluorooctan sulfonate (PFOS) in surface water from the Langat river, Peninsular Malaysia. Environ. Forensic, 13:82-92.
Zeng, H.C., Li, Y.Y., Zhang, L., Wang, Y.J., Chen, J., Xia, W. et al. (2011b). Prenatal exposure to perfluorooctanesulfonate in rat resulted in long-lasting changes of expression of synapsins and synaptophysin. Synapse, 65(3): 225-233.
Zeng, H.C., Zhang, L., Li, Y.Y., Wang, Y.J., Xia, W., Lin, Y. et al. (2011a). Inflammation-like glial response in rat brain induced by prenatal PFOS exposure. Neurotoxicology, 32(1): 130-139.
Zhang, Y.H., Wang, J., Dong, G.H., Liu, M.M., Wang, D., Zheng, L. et al. (2013). Mechanism of perfluorooctanesulfonate (PFOS)-induced apoptosis in the immunocyte. J. Immunotoxicol., 10(1): 49-58.
Zhao, L-M., Shi, L-E., Zhang, J-L., Chen, J-M., Shi, D-D., Yang, J. and Tang, Z-X. (2011). Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng., 28(3):353.
Zheng, L., Dong, G.H., Jin, Y.H. and He, Q.C. (2009). Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Arch. Toxicol., 83(7): 679-689.
Zheng, L., Dong, G.H., Zhang, Y.H., Liang, Z.F., Jin, Y.H. and He, Q.C. (2011). Type 1 and Type 2 cytokines imbalance in adult male C57BL/6 mice following a 7-day oral exposure to perfluorooctanesulfonate (PFOS). J. Immunotoxicol , 8(1): 30-38.
Appendix A: Reported full-scale drinking water treatment plant PFOS/PFOA removal data
| Water Source | Treatment TrainFootnote 2 | Influent concentration (ng/L) | Effluent concentration (ng/L) | % Removal of PFOS | Reference |
|---|---|---|---|---|---|
| Groundwater | DBF, UV, Cl2 | 10 | 9.4 | 6 | Quinones and Snyder, 2009 |
| Surface water | O3, COA/FLOC, DBF, Cl2 | 1.4 | 1.4 | 0 | Quinones and Snyder, 2009 |
| Surface water | PAC, CHLM, DBF | 1.7 | 1.9 | -12 | Quinones and Snyder, 2009 |
| Surface water | Cl2, COA/FLOC, DBF, UV | 22 | 22 | 0 | Quinones and Snyder, 2009 |
| Planned potable indirect reuse facility | MF/RO, UV/H2O2, SAT | 41 | ND | 100 | Quinones and Snyder, 2009 |
| Planned potable indirect reuse facility | Cl2, DL, SAT | 29 | 57 | -97 | Quinones and Snyder, 2009 |
| River water | RSF, O3, GAC, Cl2 | 1.0 (Summer) | 0.93 (Summer) | 7 | Takagi et al., 2008 |
| River water | RSF, O3, GAC, Cl2 | 0.87 (Summer) 3.2 (Winter) |
2.8 (Summer) 1.6 (Winter) |
-222 (summer) 5 (winter) |
Takagi et al., 2008 |
| River water | RSF, O3, GAC, Cl2 | Takagi et al., 2008 | |||
| Lake water | RSF, GAC, Cl2 | 4.6 (Summer) 4.5 (Winter) |
0.16 (Summer) <0.1 (Winter) |
97 (summer) >98 (winter) |
Takagi et al., 2008 |
| River, lake, subsoil and groundwater (7 plants) | RSF, Cl2 | 0.56 - 22 (Sum) 0.54 - 4.2 (Win) |
0.45 - 22 (Sum) 0.37 - 4.5 (Win) |
20 - 0 (summer) 31 - 0.7 (winter) |
Takagi et al., 2008 |
| River water | Membranes, Cl2 | 0.37 (Summer) 0.26 (Winter) |
0.29 (Summer) 0.20 (Winter) |
22 (summer) 23 (winter) |
Takagi et al., 2008 |
| Lake water | SSF, Cl2 | 2.7 (Summer) 1.8 (Winter) |
2.3 (Summer) 1.9 (Winter) |
15 (summer) -6 (winter) |
Takagi et al., 2008 |
| River water | COA/FLOC/SED, SF, O3, GAC, Cl2 | 1.3 (Summer) 3.3 (Winter) |
3.7 (Summer) 1.3 (Winter) |
-185 (summer) 60 (winter) |
Takagi et al., 2011 |
| River water | COA/FLOC/SED, SF, O3, GAC, Cl2 | 1.6 (Summer) 3.3 (Winter) |
2.3 (Summer) 1.7 (Winter) |
44 (summer) 48 (winter) |
Takagi et al., 2011 |
| River water | COA/FLOC/SED, SF, O3, GAC, Cl2 | 1.2 (Summer) 2.8 (Winter) |
1.6 (Summer) 1.9 (Winter) |
-33 (summer) 32 (winter) |
Takagi et al., 2011 |
| River water | SED, O3, GAC, Cl2, SF | 1.4 (Summer) 3.3 (Winter) |
2.2 (Summer) 2.0 (Winter) |
-57 (summer) 39 (winter) |
Takagi et al., 2011 |
| Lake water | COA/FLOC/SED, SF, GAC (reactivated), Cl2 | 4.4 (Summer) 4.1 (Winter) |
<0.5 (Summer) <0.5 (Winter) |
>89 (summer) >88 (winter) |
Takagi et al., 2011 |
| Ground water | UF, Cl2 | 16 | 16 | 0 | Atkinson et al., 2008 |
| Ground water | GAC (not in operation), super chlorination and dechlorination | 135 | 130 | 3 | Atkinson et al., 2008 |
| Ground water | GAC (2 parallel GAC trains each having 6 beds; contactors are mature and act as biological contactors; not been regenerated for some years), Cl2 | 42 | 45 | -7 | Atkinson et al., 2008 |
| Ground and surface water (60:40) | SSF, O3, GAC (6 beds-no regeneration for several years), Cl2using NaOCl- | 20.6 | 25 | -21 | Atkinson et al., 2008 |
| Ground water | Cl2 using NaOCl | Atkinson et al., 2008 | |||
| River water | COA/FLOC/SED, O3, GAC, RSF | 5.3 (Aug) 5.8 (Oct) |
9.4 (Aug) 6.4 (Oct) |
-77 (Aug) -10 (Oct) |
Shivakoti et al., 2010) |
| River water | COA/FLOC/SED, O3, GAC, RSF | 5.8 (Aug) 8.8 (Oct) |
3.9 (Aug) 4.2 (Oct) |
33 (Aug) 53 (Oct) |
Shivakoti et al., 2010) |
| Treated wastewater | De-nitrification, pre-O3, COA/FLOC/SED, DAF, O3, GAC (acts as biological contactor), O3 | 2,2 (Oct) 3.7 (Nov) 3.6 (Nov) |
<LOR (0.3 ng/l) (Oct) 0.6 (Nov) 0.7 (Nov) |
100 (Oct) 84 (Nov) 81 (Nov) |
Thompson et al., 2011b |
| River water | COA/FLOC/SED, RSF, Cl2 | 5.02 | 0.73 | 85 | Kunacheva et al., 2010) |
| Treated wastewater | Clarifier/lamellar settler (FeCl3&(NH4)2SO4, NaOCl addition), UF, RO, UV+H2O2, stabilization/disinfection (addition of lime, CO2, NaOCl) | 38 39 23 | <LOR (0.5 ng/L) ND <LOR (0.2 ng/L | 100 100 100 | Thompson et al., 2011 |
| River water | COA/FLOC, RSF, O3, GAC, SSF | 8.2 | <0.23 | <97 | Eschauzer et al., 2012 |
| River water | Cl2, COA/FLOC, RSF, O3, GAC | 116 | 33 | 69 | Flores et al., 2013 |
| River water | Cl2, COA/FLOC, RSF, O3, GAC, UF, RO | 86 | 13 | 86 | Flores et al., 2013 |
Appendix B: List of Acronyms
- AFFF
- aqueous film-forming foam
- ALT
- alanine transaminase
- BMD
- benchmark dose
- BMDL
- lower confidence limit on the benchmark dose
- BMDL10
- lower 95% confidence limit on the benchmark dose for a 10% response
- BV
- bed volume
- CAS
- Chemical Abstracts Service
- CI
- confidence interval
- CSAF
- chemical specific adjustment factor
- DI
- direct injection
- DL
- detection limit
- EBCT
- empty bed contact time
- ESI
- electrospray ionization
- GAC
- granular activated carbon
- GD
- gestational day
- GM
- geometric mean
- HBV
- health-based value
- HPLC
- high performance liquid chromatography
- IT
- ion-trap
- LC
- liquid chromatograph
- LOAEL
- lowest-observed-adverse-effect level
- LOD
- limit of detection
- LOQ
- limit of quantitation
- LLE
- liquid-liquid extraction
- MAC
- maximum acceptable concentration
- MDL
- method detection limit
- MOA
- mode of action
- MRL
- minimum reporting level
- MS/MS
- tandem mass spectrometry
- NF
- nanofiltration
- NOAEL
- no-observed-adverse-effect level
- NOM
- natural organic matter
- PAC
- powdered activated carbon
- PBPK
- Physiologically-based pharmacokinetic
- PEFT
- polytetrafluoroethylene
- PFAA
- perfluorinated alkyl acid
- PFAS
- perfluoroalkyl substance
- PFCA
- long-chain perfluorocarboxylic acids
- PFOA
- perfluorooctanoic acid
- PFOS
- perfluorooctane sulfonate
- PND
- postnatal day
- POD
- point of departure
- PODHEQ
- human-equivalent points-of-departure
- PTFE
- polytetrafluoroethylene
- RBF
- river bank filtration
- RO
- reverse osmosis
- SPE
- solid phase extraction
- TDI
- tolerable daily intake
- TDS
- total diet study
- UCMR3
- third Unregulated Contaminant Monitoring Rule
Appendix C: Provincial and territorial anticipated impacts
Please note that this information is not available in both official languages because the source of the information is not subject to the Official Languages Act.Prince Edward Island
No impact paragraph has been provided by the province.Newfoundland and Labrador
Newfoundland and Labrador has not conducted monitoring for PFOS. Of the 305 surface water sources in the province, 258 (85%) are protected under the Water Resources Act. Of the 198 groundwater sources in the province, 59 (30%) are protected under the Water Resources Act. This provides for an extensive source water protection program that reduces the risk of contamination for drinking water sources.As noted in the Technical Document, PFOS does not naturally occur in the environment and would only be relevant to areas that are subjected to industrial activities. The main cause for concern in NL would be the short-term use of fire-fighting foams for either property or forest fires.
The majority of Newfoundland and Labrador's drinking water systems do not have any treatment other than disinfection as 68% of the systems service very small populations (less than 500 people). Due to this fact, the ability for the majority of communities to treat for PFOS contamination would be limited. The cost would have to be assessed on a case by case basis but the risk of PFOS formation would be low to the protection of public water supplies.
The province will consider monitoring for PFOS if/when fire-fighting foams are utilized in public drinking water supply areas and is deemed relevant and if/when fire-fighting foams are utilized in single-dwelling homes on private wells when deemed relevant.
Nova Scotia
No impact paragraph has been provided by the province.New Brunswick
PFOS is not monitored in New Brunswick and it is not anticipated that this Guideline would have any impact on regulated drinking water facilities. However, we would have to consult with stakeholders to determine if source water characterization would be necessary and the associated impacts related to costs and the availability of analytical testing. If it was found in source waters this could result in an impact to that facility in terms of additional monitoring or treatment requirements.Quebec
Au Québec, étant donné que le PFOS ne fait pas l'objet d'une norme au Règlement sur la qualité de l'eau potable, les résultats disponibles découlent des campagnes d'échantillonnage réalisées par le Ministère dans le cadre Programme de surveillance de la qualité de l'eau potable.Les résultats disponibles indiquent de très faibles concentrations mesurées de ce paramètre, très en-deçà de la CMA proposée. Plus précisément, en 2007 et 2008, 84 analyses de PFOS ont été réalisées à l'eau traitée de 7 installations de production d'eau potable alimentées en eau de surface. Ces installations avaient été sélectionnées puisqu'elles étaient localisées à des endroits où l'on était susceptible de détecter ces substances (ex. : villes d'importance en amont, présence d'industries de textiles). Les résultats indiquent que 52 % des échantillons prélevés ont présenté un résultat supérieur à la limite de détection et le PFOS a été détecté dans 86 % des installations échantillonnées. La concentration maximale mesurée était de 0,012 µg/L, ce qui est bien inférieur à la CMA proposée de 0,6 µg/L.
Considérant les résultats d'analyse disponibles, les impacts attendus de l'ajout d'une norme pour le PFOS à la réglementation, en fonction de la recommandation publiée par Santé Canada, seraient faibles.